Foraging Behaviour and Sensory Ecology of the Bat-Eared Fox (Otocyon Megalotis)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
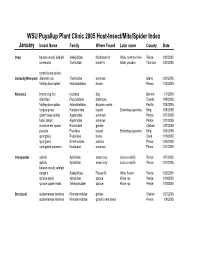
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

Sultan Qaboos University Journal for Scientific Research
Agricultural and Marine Sciences, 10(1):33-40 (2005) ©2005 Sultan Qaboos University Identification, Geographical Distribution and Hosts of Subterranean Termites in the United Arab Emirates Arid Ecosystem W. Kaakeh Department of Arid Land Agriculture, College of Food Systems, P. O. Box 17555, United Arab Emirates University, Al-Ain, United Arab Emirates وﻟﯿﺪ ﻛﻌﻚ اﻟﺨﻼﺻﺔ: ﺗﻢ ﺗﻌﺮﻳﻒ ﺳﺘﺔ أﻧﻮاع ﻣﻦ اﻟﻨﻤﻞ اﻷﺑﯿﺾ (اﻷرﺿﺔ) ﺗﺎﺑﻌﺔ إﻟﻰ ﺧﻤﺴﺔ أﺟﻨﺎس وﺛﻼث ﻓﺼﺎﺋﻞ (ھﻮدوjرﻣﯿﺘﯿﺪي Hodotermitidae، راﻳﻨﻮﺗﺮﻣﯿﺘﯿﺪي Rhinotermi،Rhinotermitidaetidae، وﺗﺮﻣﯿﺘﯿﺪي T(Termitidaeermitidae) ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وأﻧﻮاع اﻷرﺿﺔ اﻟﺘﻲ ﺗﻢ ﺗﺴﺠﯿﻠﮭﺎ ھﻲ اﻷرﺿﺔ اﻟﺤﺎﺻﺪة أو اﻷرﺿﺔ اﻟﻼﺷﻮﻛﯿﺔ Anacanthotermes ochraceusochraceus (Burmeister(Burmeister) و Anacanthotermes ubachi (Navas(Navas)، وأرﺿﺔ اﻟﺮﻣﻞ اﻟﺜﻐﺮﻳﺔ Psammotermes hypostomahypostoma (Desneux)، واﻷرﺿﺔ اﻟﺸﻤﻌﯿﺔ اﻟﺼﻐﯿﺮة MicrocerotermesMicrocerotermes diversusdiversus Silvestri))، واﻷرﺿﺔ اﻟﻨﺠﺪﻳﺔ اﻟﺪﻗﯿﻘﺔ Microtermes najdensis (Harris) ، واﻷرﺿﺔ Heterotermes aethiopicus (Sjostedt)، وﺑﺎﺳﺘﺜﻨﺎء اﻟﻨﻮع H. aethiopicus، ﻓﺈﻧﻪ ﺗﻢ ﺗﺴﺠﯿﻞ اﻷﻧﻮاع اﻟﺨﻤﺴﺔ اﻷﺧﺮى ﻟﻠﻤﺮة اﻷوﻟﻰ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وﺗﻌﯿﺶ ﻛﻞ اﻷﻧﻮاع ﺗﺤﺖ اﻷرض وﺗﺼﻞ إﻟﻰ ﻣﺼﺎدر اﻟﻐﺬاء اﻟﺨﺸﺒﯿﺔ ﻣﻦ ﺧﻼل أﻧﻈﻤﺔ اﻷﻧﻔﺎق اﻟﻄﯿﻨﯿﺔ. وﻗﺪ وﺟﺪت اﻷرﺿﺔ ﻓﻲ ﻣﻨﺎﻃﻖ ﻣﺨﺘﻠﻔﺔ ﻣﻦ اﻟﺪوﻟﺔ واﻟﺘﻲ ﺗﺘﻤﯿﺰ ﺑﺎﺧﺘﻼف ﻇﺮوﻓﮭﺎ اﻟﻤﻨﺎﺧﯿﺔ وﻏﻄﺎءھﺎ اﻟﻨﺒﺎﺗﻲ وﻧﻮع ﺗﺮﺑﺘﮭﺎ. وﺗﻔﻀﻞ اﻷرﺿﺔ اﻟﺘﻐﺬﻳﺔ ﻋﻠﻰ اﻟﻌﻮاﺋﻞ اﻟﺤﯿﺔ أو اﻟﻤﯿﺘﺔ أو اﻟﻤﺘﻌﻔﻨﺔ، ﺑﺎﻹﺿﺎﻓﺔ إﻟﻰ اﻟﻤﻮاد ﻏﯿﺮ اﻟﺴﯿﻠﯿﻠﻮزﻳﺔ. وﻣﻦ أﻛﺜﺮ أﻧﻮاع اﻷرﺿﺔ ًﺗﻮزﻳﻌﺎ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة ھﻲ A. ochraceusochraceus وﺗﺘﺒﻌﮭﺎ ﻛﻞ ﻣﻦ P.P. hypostomahypostoma وdiversusوM. diversus. وﻗﺪ اﺧﺘﻠﻒ ﺗﻮزﻳﻊ اﻷﻧﻮاع اﻟﺴﺘﺔ ﺿﻤﻦ -

Blattodea: Hodotermitidae) and Its Role As a Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia
Article Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia Reem Alajmi 1,*, Rewaida Abdel-Gaber 1,2,* and Noura AlOtaibi 3 1 Zoology Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia 2 Zoology Department, Faculty of Science, Cairo University, Cairo 12613, Egypt 3 Department of Biology, Faculty of Science, Taif University, Taif 21974, Saudi Arabia; [email protected] * Correspondence: [email protected] (R.A.), [email protected] (R.A.-G.) Received: 28 December 2018; Accepted: 3 February 2019; Published: 8 February 2019 Abstract: Termites are social insects of economic importance that have a worldwide distribution. Identifying termite species has traditionally relied on morphometric characters. Recently, several mitochondrial genes have been used as genetic markers to determine the correlation between different species. Heavy metal accumulation causes serious health problems in humans and animals. Being involved in the food chain, insects are used as bioindicators of heavy metals. In the present study, 100 termite individuals of Anacanthotermes ochraceus were collected from two Saudi Arabian localities with different geoclimatic conditions (Riyadh and Taif). These individuals were subjected to morphological identification followed by molecular analysis using mitochondrial 12S rRNA gene sequence, thus confirming the morphological identification of A. ochraceus. Furthermore, a phylogenetic analysis was conducted to determine the genetic relationship between the acquired species and other termite species with sequences previously submitted in the GenBank database. Several heavy metals including Ca, Al, Mg, Zn, Fe, Cu, Mn, Ba, Cr, Co, Be, Ni, V, Pb, Cd, and Mo were measured in both collected termites and soil samples from both study sites. -

The Phylogeny of Termites
Molecular Phylogenetics and Evolution 48 (2008) 615–627 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev The phylogeny of termites (Dictyoptera: Isoptera) based on mitochondrial and nuclear markers: Implications for the evolution of the worker and pseudergate castes, and foraging behaviors Frédéric Legendre a,*, Michael F. Whiting b, Christian Bordereau c, Eliana M. Cancello d, Theodore A. Evans e, Philippe Grandcolas a a Muséum national d’Histoire naturelle, Département Systématique et Évolution, UMR 5202, CNRS, CP 50 (Entomologie), 45 rue Buffon, 75005 Paris, France b Department of Integrative Biology, 693 Widtsoe Building, Brigham Young University, Provo, UT 84602, USA c UMR 5548, Développement—Communication chimique, Université de Bourgogne, 6, Bd Gabriel 21000 Dijon, France d Muzeu de Zoologia da Universidade de São Paulo, Avenida Nazaré 481, 04263-000 São Paulo, SP, Brazil e CSIRO Entomology, Ecosystem Management: Functional Biodiversity, Canberra, Australia article info abstract Article history: A phylogenetic hypothesis of termite relationships was inferred from DNA sequence data. Seven gene Received 31 October 2007 fragments (12S rDNA, 16S rDNA, 18S rDNA, 28S rDNA, cytochrome oxidase I, cytochrome oxidase II Revised 25 March 2008 and cytochrome b) were sequenced for 40 termite exemplars, representing all termite families and 14 Accepted 9 April 2008 outgroups. Termites were found to be monophyletic with Mastotermes darwiniensis (Mastotermitidae) Available online 27 May 2008 as sister group to the remainder of the termites. In this remainder, the family Kalotermitidae was sister group to other families. The families Kalotermitidae, Hodotermitidae and Termitidae were retrieved as Keywords: monophyletic whereas the Termopsidae and Rhinotermitidae appeared paraphyletic. -

Mcabee Fossil Site Assessment
1 McAbee Fossil Site Assessment Final Report July 30, 2007 Revised August 5, 2007 Further revised October 24, 2008 Contract CCLAL08009 by Mark V. H. Wilson, Ph.D. Edmonton, Alberta, Canada Phone 780 435 6501; email [email protected] 2 Table of Contents Executive Summary ..............................................................................................................................................................3 McAbee Fossil Site Assessment ..........................................................................................................................................4 Introduction .......................................................................................................................................................................4 Geological Context ...........................................................................................................................................................8 Claim Use and Impact ....................................................................................................................................................10 Quality, Abundance, and Importance of the Fossils from McAbee ............................................................................11 Sale and Private Use of Fossils from McAbee..............................................................................................................12 Educational Use of Fossils from McAbee.....................................................................................................................13 -

Du Plessis 2018 Phd Gathering the Kalahari
UC Santa Cruz UC Santa Cruz Electronic Theses and Dissertations Title Gathering the Kalahari: Tracking Landscapes in Motion Permalink https://escholarship.org/uc/item/7b98v9k6 Author du Plessis, Pierre Louis Publication Date 2018 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA SANTA CRUZ GATHERING THE KALAHARI: TRACKING LANDSCAPES IN MOTION A dissertation submitted in partial satisfaction of the requirements for the degree of DOCTOR OF PHILOSOPHY in ANTHROPOLOGY by Pierre L. du Plessis June 2018 The Dissertation of Pierre du Plessis is approved: _____________________________________ Professor Anna Tsing, chair ____________________________________ Professor Andrew Mathews _____________________________________ Professor Mayanthi Fernando ____________________________ Tyrus Miller Vice Provost and Dean of Graduate Studies Copyright © by Pierre L. du Plessis 2018 Table of Contents Table of Figures ............................................................................................................................ v Abstract ........................................................................................................................................ vi Acknowledgements ................................................................................................................... ix Introduction. “Keep on Tracking:” Finding Openings in the Kalahari Desert ............ 1 Part One. Opening: An introduction in four parts ................................................................................. -

Very Similar to 'M.' Limai, Except for the Distinctly Larger Size, and Thus
Insects of the Crato Formation 249 Comment: very similar to ‘M.’ limai, except for the distinctly larger size, and thus probably belonging to the same new genus. It has not been possible to determine whether this new taxon is conspecific with the undescribed new genus and species mentioned by Vrˇsansk´y (2004) or instead represents a third blattellid taxon from Crato. Blattidae The presence of the Blattidae in the Crato Formation was first noted by Mendes (1993), who recognized that Mesoblattinopsis schneideri Pinto, 1989 was a blattid. Mesoblattinopsis schneideri Pinto, 1989 Comment: two further new species of Mesoblattinopsis are reported by Mendes (1997b). Family incertae sedis Unnamed new genus and species B Material: three specimens with nos SMNS 66321, SMNS 66308 (Figure 11.23h) and SMNS 66309. Diagnosis: body length about 8.7–9.5 mm; shape of body longish oval; antennae about as long as body; pronotum much broader than head (width 3.0–3.7 mm, thus 180–195% of head width), posteriorly broader than anteriorly, but with narrower lateral lobes than the new blattellid species mentioned above; forewing venation unknown, but with a broad costal margin; cerci with about 10 segments. 11.9 Isoptera: termites G¨unter Bechly There are about 2,800 Recent and about 130 fossil termite species, usually classified in seven families (see below). Termites are relatively small insects with a body length that is usually much less than 3 cm. They are soft-bodied and often called white ants because they are small colonial insects of more or less whitish color. They are, however, completely unrelated to ants, but closely related to cockroaches and mantids. -

Order Blattodea*
Zootaxa 3703 (1): 046–048 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3703.1.10 http://zoobank.org/urn:lsid:zoobank.org:pub:72196B26-433A-4816-90B3-9EC15495E1B4 Order Blattodea* GEORGE BECCALONI1 & PAUL EGGLETON2 1Curator of orthopteroid insects, Life Sciences Department, Terrestrial Invertebrates Division, The Natural History Museum, London SW7 5BD, UK; email: [email protected] 2Merit Researcher, Life Sciences Department, Terrestrial Invertebrates Division, The Natural History Museum, London SW7 5BD, UK * In: Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-level Classification and Survey of Taxonomic Richness (Addenda 2013). Zootaxa, 3703, 1–82. Abstract The Blattodea comprise the termites (epifamily Termitoidae only) and the cockroaches (all other taxa). 7570 living species of Blattodea are currently recognised, of which 2929 are termites (Krishna et al. 2013) and 4641 are cockroaches (Beccaloni 2007) . Key words: Blattodea, cockroaches, termites, classification, diversity Introduction The Blattodea comprise the termites (epifamily Termitoidae only) and the cockroaches (all other taxa). Beccaloni and Eggleton (2011) recognized 7314 extant named species of Blattodea, including 2692 termites and 4622 cockroaches and. In this update, 7570 living species of Blattodea are currently recognised, of which 2929 are termites (Krishna et al. 2013) and 4641 are cockroaches (Beccaloni 2007) . Inward, Beccaloni & Eggleton (2007) and subsequent phylogenetic studies (Legendre et al. 2008; Ware et al. 2008; Cameron et al. 2012; Djernaes et al. 2012; Xiao et al. 2012) have confirmed that the termites and the cockroach family Cryptocercidae are sister groups and that this clade is nested within the Blattodea. -

Genetic Diversity of the Genus Terfezia (Pezizaceae, Pezizales): New Species and New Record from North Africa
Phytotaxa 334 (2): 183–194 ISSN 1179-3155 (print edition) http://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2018 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.334.2.7 Genetic diversity of the genus Terfezia (Pezizaceae, Pezizales): New species and new record from North Africa FATIMA EL-HOUARIA ZITOUNI-HAOUAR1*, JUAN RAMÓN CARLAVILLA2, GABRIEL MORENO2, JOSÉ LUIS MANJÓN2 & ZOHRA FORTAS1 1 Laboratoire de Biologie des Microorganismes et de Biotechnologie, Département de Biotechnologie, Faculté des Sciences de la nature et de la vie, Université d’Oran 1 Ahmed Ben Bella, Algeria 2 Departamento Ciencias de la Vida, Facultad de Biología, Universidad de Alcalá, 28805 Alcalá de Henares, Madrid, Spain * Corresponding author: [email protected] Abstract Morphological and phylogenetic analyses of large ribosomal subunit (28S rDNA) and internal transcribed spacer (ITS rDNA) of Terfezia samples collected from several bioclimatic zones in Algeria and Spain revealed the presence of six dis- tinct Terfezia species: T. arenaria, T. boudieri, T. claveryi; T. eliocrocae (reported here for the first time from North Africa), T. olbiensis, and a new species, T. crassiverrucosa sp. nov., proposed and described here, characterized by its phylogenetic position and unique combination of morphological characters. A discussion on the unresolved problems in the taxonomy of the spiny-spored Terfezia species is conducted after the present results. Key words: desert truffles, Pezizaceae, phylogeny, taxonomy Introduction The genus Terfezia (Tul. & C.Tul.) Tul. & C. Tul. produce edible hypogeous ascomata growing mostly in arid and semi-arid ecosystems, although they can be found also in a wide range of habitats, such as temperate deciduous forests, conifer forests, prairies, or even heath lands (Moreno et al. -

The Nature of Alarm Communication in Constrictotermes Cyphergaster (Blattodea: Termitoidea: Termitidae): the Integration of Chemical and Vibroacoustic Signals Paulo F
© 2015. Published by The Company of Biologists Ltd | Biology Open (2015) 4, 1649-1659 doi:10.1242/bio.014084 RESEARCH ARTICLE The nature of alarm communication in Constrictotermes cyphergaster (Blattodea: Termitoidea: Termitidae): the integration of chemical and vibroacoustic signals Paulo F. Cristaldo1,*, Vojtĕch Jandák2, Kateřina Kutalová3,4,5, Vinıciuś B. Rodrigues6, Marek Brothánek2, Ondřej Jiřıć̌ek2, Og DeSouza6 and Jan Šobotnıḱ3 ABSTRACT INTRODUCTION Predation and competition have long been considered major ecological Alarm signalling is of paramount importance to communication in all factors structuring biotic communities (e.g. Shurin and Allen, 2001). In social insects. In termites, vibroacoustic and chemical alarm signalling many taxa, a wide range of defensive strategies have evolved helping are bound to operate synergistically but have never been studied organisms to deal with costs imposed by such interactions. Strategies simultaneously in a single species. Here, we inspected the functional consist in combining mechanical (biting, kicking, fleeing) and/or significance of both communication channels in Constrictotermes chemical (toxic or repellent compounds) arsenal with an effective cyphergaster (Termitidae: Nasutitermitinae), confirming the hypothesis alarm communication. The ability to give and perceive conspecific that these are not exclusive, but rather complementary processes. In signalling about imminent threats has attained maximum complexity natural situations, the alarm predominantly attracts soldiers, which in social groups, such as vertebrates (Manser, 2001, 2013) and insects actively search for the source of a disturbance. Laboratory testing (Blum, 1985; Hölldobler, 1999; Hölldobler and Wilson, 2009). revealed that the frontal gland of soldiers produces a rich mixture of In termites, one facet of such complexity is represented by the terpenoid compounds including an alarm pheromone. -

Duke University Dissertation Template
Systematics, Phylogeography and Ecology of Elaphomycetaceae by Hannah Theresa Reynolds Department of Biology Duke University Date:_______________________ Approved: ___________________________ Rytas Vilgalys, Supervisor ___________________________ Marc Cubeta ___________________________ Katia Koelle ___________________________ François Lutzoni ___________________________ Paul Manos Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biology in the Graduate School of Duke University 2011 iv ABSTRACTU Systematics, Phylogeography and Ecology of Elaphomycetaceae by Hannah Theresa Reynolds Department of Biology Duke University Date:_______________________ Approved: ___________________________ Rytas Vilgalys, Supervisor ___________________________ Marc Cubeta ___________________________ Katia Koelle ___________________________ François Lutzoni ___________________________ Paul Manos An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Department of Biology in the Graduate School of Duke University 2011 Copyright by Hannah Theresa Reynolds 2011 Abstract This dissertation is an investigation of the systematics, phylogeography, and ecology of a globally distributed fungal family, the Elaphomycetaceae. In Chapter 1, we assess the literature on fungal phylogeography, reviewing large-scale phylogenetics studies and performing a meta-data analysis of fungal population genetics. In particular, we examined