The Diversification of Termites: Inferences from a Complete Species-Level Phylogeny
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evolution and Ecology of Termite Nesting Behavior and Its Impact On
1 Evolution and Ecology of Termite Nesting Behavior and Its Impact on Disease Susceptibility A dissertation presented by Marielle Aimée Postava-Davignon to The Department of Biology In partial fulfillment of the requirements for the degree of Doctor of Philosophy in the field of Biology Northeastern University Boston, Massachusetts April, 2010 2 Evolution and Ecology of Termite Nesting Behavior and Its Impact on Disease Susceptibility by Marielle Aimée Postava-Davignon ABSTRACT OF DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biology in the Graduate School of Arts and Sciences of Northeastern University, April, 2010 3 Abstract Termites construct nests that are often structurally species-specific. They exhibit a high diversity of nest structures, but their nest evolution is largely unknown. Current hypotheses for the factors that influenced nest evolution include adaptations that improved nest thermoregulation, defense against predators, and competition for limited nest sites. Studies have shown a lower prevalence of pathogens and parasites in arboreal nesting animal species compared to ground nesters. Nest building behavior is plastic and can adapt to changing environments. As termites can detect and avoid pathogens, I hypothesized that the evolution of arboreal termite nests was an adaptation to avoid infection. To test this, bacteria and fungi from nest cores, trails, and surrounding soils of the arboreal nesting Nasutitermes acajutlae were cultured. Abiotic factors such as temperature, relative humidity, and light were measured to elucidate how they influenced the interactions between termites and microbes. Fungi associated with N. acajutlae were identified to determine the potential pathogenic pressures these termites encounter in their nest as compared to the external environment. -

AUSTRALIAN TERMITOPHILES ASSOCIATED with MICROCEROTERMES (Isoptera: Amitermitinae) I
Pacific Insects 12 (1): 9-15 20 May 1970 AUSTRALIAN TERMITOPHILES ASSOCIATED WITH MICROCEROTERMES (Isoptera: Amitermitinae) I. A new Subtribe, genus, and species (Coleoptera, Staphylinidae) with notes on their behavior1 By David H. Kistner2 Abstract: A new Subtribe (Microceroxenina) of the tribe Athetini is described. The single included genus and species (both new) is Microceroxenus alzadae which was cap tured with Microcerotermes turneri in North Queensland. Behavioral observations are presented which support the interpretation that Microceroxenus is well-integrated into the social life of the termites. Observations of the release of alates by the host ter mites are presented which support the interpretation that the release of alates in these termites is simultaneous among colonies in a given area, is of short duration, and oc curs rather infrequently. Not many species of termitophiles have been found with termites of the genus Micro cerotermes Silvestri (Amitermitinae) or even from the genera related to Microcerotermes such as Amphidotermes or Globitermes (Ahmad 1950). Only 1 species of staphylinid has been previously recorded and that species is Termitochara kraatzi Wasmann which was collected with Microcerotermes sikorae (Wasmann) from Madagascar (Seevers 1957). The same species of termitophile has also been recorded from a nest of Capritermes capricor- nis (Wasmann), which belongs to an entirely different subfamily (Termitinae), by Was mann (1893). No one really believes either of these termites is the true host of the species as the nearest relatives of Termitochara are found principally with the Nasutiter- mitinae. It was therefore a real pleasure to open up a Microcerotermes nest and find numerous staphylinids there, particularly when opening up nests of the same genus in Africa had never yielded any staphylinids. -

The Termite by Ogden Nash
Biological studies on two European termite species: establishment risk in the UK Laetitia Virginie Laine B.Sc. M.Sc. D.I.C. A thesis submitted for the degree of Doctor of Philosophy of the University of London November 2002 Department of Biological Sciences, Imperial College, Silwood Park, Ascot, SL5 7PY, Berkshire Abstract The discovery of an accidental introduction of termites into Devon in 1994 generated great interest as termites were previously thought to be unable to establish in the UK due to unfavourable climatic conditions. Information about the species present in Devon, Reticulitermes grassei, was found to be lacking and the present study was undertaken to determine the importance of various abiotic and biotic factors in establishment of this species. The factors included in the study were the minimum termite number for establishment, the consumption of wood and its effect on survival and temperature and soil type. A review of the literature was also conducted, detailing the problems with the taxonomy of this termite genus, their present distribution pattern and the life cycle of Reticulitermes species. Two populations of both R. grassei and R. santonensis were studied. The effect of the minimum termite number was found to be significant in both laboratory and field conditions. However, survival decreased in the laboratory and increased in the field with increased number of termites. Consumption experiments were performed using blocks of Scots pine, beech and oak. In most cases termite populations were found to consume and survive best on oak. Consumption was also tested on live seedlings but these results were inconclusive. Survival was observed to increase with increased temperature. -
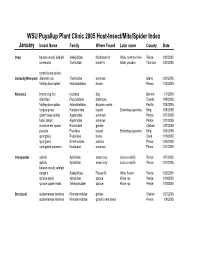
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

Stable Isotope Methods in Biological and Ecological Studies of Arthropods
eea_572.fm Page 3 Tuesday, June 12, 2007 4:17 PM DOI: 10.1111/j.1570-7458.2007.00572.x Blackwell Publishing Ltd MINI REVIEW Stable isotope methods in biological and ecological studies of arthropods CORE Rebecca Hood-Nowotny1* & Bart G. J. Knols1,2 Metadata, citation and similar papers at core.ac.uk Provided by Wageningen University & Research Publications 1International Atomic Energy Agency (IAEA), Agency’s Laboratories Seibersdorf, A-2444 Seibersdorf, Austria, 2Laboratory of Entomology, Wageningen University and Research Centre, P.O. Box 8031, 6700 EH Wageningen, The Netherlands Accepted: 13 February 2007 Key words: marking, labelling, enrichment, natural abundance, resource turnover, 13-carbon, 15-nitrogen, 18-oxygen, deuterium, mass spectrometry Abstract This is an eclectic review and analysis of contemporary and promising stable isotope methodologies to study the biology and ecology of arthropods. It is augmented with literature from other disciplines, indicative of the potential for knowledge transfer. It is demonstrated that stable isotopes can be used to understand fundamental processes in the biology and ecology of arthropods, which range from nutrition and resource allocation to dispersal, food-web structure, predation, etc. It is concluded that falling costs and reduced complexity of isotope analysis, besides the emergence of new analytical methods, are likely to improve access to isotope technology for arthropod studies still further. Stable isotopes pose no environmental threat and do not change the chemistry or biology of the target organism or system. These therefore represent ideal tracers for field and ecophysiological studies, thereby avoiding reductionist experimentation and encouraging more holistic approaches. Con- sidering (i) the ease with which insects and other arthropods can be marked, (ii) minimal impact of the label on their behaviour, physiology, and ecology, and (iii) environmental safety, we advocate more widespread application of stable isotope technology in arthropod studies and present a variety of potential uses. -

Diversity of Ants and Termites of the Botanical Garden of the University of Lomé, Togo
insects Article Diversity of Ants and Termites of the Botanical Garden of the University of Lomé, Togo Boris Dodji Kasseney 1,* , Titati Bassouo N’tie 1, Yaovi Nuto 1, Dekoninck Wouter 2 , Kolo Yeo 3 and Isabelle Adolé Glitho 1 1 Laboratoire d’Entomologie Appliquée, Département de Zoologie et de Biologie Animale, Université de Lomé, 01 BP 1515, Lomé 01, Lome 151, Togo 2 RBINS Scientific Service Heritage/O.D. Taxonomy and Phylogeny, Curator Entomology Collections, Vautierstraat 29, 1000 Brussels, Belgium 3 Université Nangui Abrogoua, 02 BP 801 Abidjan 02, Abidjan, Côte d’Ivoire * Correspondence: [email protected]; Tel.: +228-906-156-74 Received: 6 June 2019; Accepted: 22 July 2019; Published: 23 July 2019 Abstract: Ants and termites are used as bioindicators in many ecosystems. Little knowledge is available about them in Togo, especially ants. This study aimed to find out how ants and termites could be used to assess the restoration of former agricultural land. These insect groups were sampled within six transects of 50 2 m2 (using pitfall traps, monoliths, baits for ants and hand sampling for × termites) in two consecutive habitats: open area (grassland) and covered area (an artificial forest). Seventeen termite species and 43 ant species were collected. Seven ant species were specific to the covered area against four for the open area, while four unshared species of termite were found in the open area against three in the covered area. The presence of unshared species was linked to vegetation, as Trinervitermes (Holmgren, 1912), a grass feeding termite, was solely found in open area. Also, for some ant species like Cataulacus traegaordhi (Santschi, 1914), Crematogaster (Lund, 1831) species, Oecophylla longinoda (Latreille, 1802) and Tetraponera mocquerysi (Brown, 1960), all arboreal species, vegetation was a determining factor for their presence. -

Treatise on the Isoptera of the World Kumar
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by American Museum of Natural History Scientific Publications KRISHNA ET AL.: ISOPTERA OF THE WORLD: 7. REFERENCES AND INDEX7. TREATISE ON THE ISOPTERA OF THE WORLD 7. REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA, AND MICHAEL S. ENGEL A MNH BULLETIN (7) 377 2 013 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY TREATISE ON THE ISOPTERA OF THE WORLD VolUME 7 REFERENCES AND INDEX KUMAR KRISHNA, DAVID A. GRIMALDI, VALERIE KRISHNA Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192 AND MICHAEL S. ENGEL Division of Invertebrate Zoology, American Museum of Natural History Central Park West at 79th Street, New York, New York 10024-5192; Division of Entomology (Paleoentomology), Natural History Museum and Department of Ecology and Evolutionary Biology 1501 Crestline Drive, Suite 140 University of Kansas, Lawrence, Kansas 66045 BULLETIN OF THE AMERICAN MUSEUM OF NATURAL HISTORY Number 377, 2704 pp., 70 figures, 14 tables Issued April 25, 2013 Copyright © American Museum of Natural History 2013 ISSN 0003-0090 2013 Krishna ET AL.: ISOPtera 2435 CS ONTENT VOLUME 1 Abstract...................................................................... 5 Introduction.................................................................. 7 Acknowledgments . 9 A Brief History of Termite Systematics ........................................... 11 Morphology . 44 Key to the -

Blattabacterium Genome: Structure, Function, and Evolution Austin Alleman
University of Texas at Tyler Scholar Works at UT Tyler Biology Theses Biology Spring 6-5-2014 Blattabacterium Genome: Structure, Function, and Evolution Austin Alleman Follow this and additional works at: https://scholarworks.uttyler.edu/biology_grad Part of the Biology Commons Recommended Citation Alleman, Austin, "Blattabacterium Genome: Structure, Function, and Evolution" (2014). Biology Theses. Paper 20. http://hdl.handle.net/10950/215 This Thesis is brought to you for free and open access by the Biology at Scholar Works at UT Tyler. It has been accepted for inclusion in Biology Theses by an authorized administrator of Scholar Works at UT Tyler. For more information, please contact [email protected]. BLATTABACTERIUM GENOME: STRUCTURE, FUNCTION, AND EVOLUTION by AUSTIN ALLEMAN A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science Department of Biology Srini Kambhampati, Ph. D., Committee Chair College of Arts and Sciences The University of Texas at Tyler May 2014 © Copyright by Austin Alleman 2014 All rights reserved Acknowledgements I would like to thank the Sam A. Lindsey Endowment, without whose support this research would not have been possible. I would also like to thank my research advisor, Dr. Srini Kambhampati, for his knowledge, insight, and patience; and my committee, Dr. John S. Placyk, Jr. and Dr. Blake Bextine, for their assistance and feedback. “It is the supreme art of the teacher to awaken joy in creative expression and knowledge.” --Albert Einstein Table of Contents Chapter -

Characterization of the 12S Rrna Gene Sequences of the Harvester
Article Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia Reem Alajmi 1,*, Rewaida Abdel-Gaber 1,2,* and Noura AlOtaibi 3 1 Zoology Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia 2 Zoology Department, Faculty of Science, Cairo University, Cairo 12613, Egypt 3 Department of Biology, Faculty of Science, Taif University, Taif 21974, Saudi Arabia; [email protected] * Correspondence: [email protected] (R.A.), [email protected] (R.A.-G.) Received: 28 December 2018; Accepted: 3 February 2019; Published: 8 February 2019 Abstract: Termites are social insects of economic importance that have a worldwide distribution. Identifying termite species has traditionally relied on morphometric characters. Recently, several mitochondrial genes have been used as genetic markers to determine the correlation between different species. Heavy metal accumulation causes serious health problems in humans and animals. Being involved in the food chain, insects are used as bioindicators of heavy metals. In the present study, 100 termite individuals of Anacanthotermes ochraceus were collected from two Saudi Arabian localities with different geoclimatic conditions (Riyadh and Taif). These individuals were subjected to morphological identification followed by molecular analysis using mitochondrial 12S rRNA gene sequence, thus confirming the morphological identification of A. ochraceus. Furthermore, a phylogenetic analysis was conducted to determine the genetic relationship between the acquired species and other termite species with sequences previously submitted in the GenBank database. Several heavy metals including Ca, Al, Mg, Zn, Fe, Cu, Mn, Ba, Cr, Co, Be, Ni, V, Pb, Cd, and Mo were measured in both collected termites and soil samples from both study sites. -

Discovery of a Cryptic Termite Genus, Stylotermes (Isoptera: Stylotermitidae), in Taiwan, with the Description of a New Species
Annals of the Entomological Society of America, 110(4), 2017, 360–373 doi: 10.1093/aesa/sax034 Advance Access Publication Date: 23 March 2017 Research Research article Discovery of a Cryptic Termite Genus, Stylotermes (Isoptera: Stylotermitidae), in Taiwan, With the Description of a New Species Wei-Ren Liang, Chia-Chien Wu, and Hou-Feng Li1 Department of Entomology, National Chung Hsing University, 145 Xingda Rd., Taichung 40227, Taiwan ([email protected]; [email protected]; [email protected]), and 1Corresponding author, e-mail: [email protected] Subject Editor: Allen Szalanski Received 18 October 2016; Editorial decision 11 January 2017 Abstract The termite family Stylotermitidae consists of a single extant genus, Stylotermes, present only in eastern Asia. Stylotermes has distinctively trimerous tarsi and is considered an intermediate between the Rhinotermitidae and Kalotermitidae families. The present study reports the first discovery of the Stylotermitidae family in Taiwan. On the basis of a comparison between the Taiwanese samples and the original descriptions of all the other 44 Stylotermes species, a new species collected from eastern Taiwan is described as Stylotermes halumi- cus sp. nov. This study is the first to report the gene sequence and detailed morphological descriptions of the winged imago, soldier, and worker of a single Stylotermes sp. Furthermore, a preliminary review of the Stylotermes taxonomy is provided. Key words: Stylotermitidae, wood-feeding termite, Taiwan, 16S, COII Holmgren and Holmgren (1917) established a new genus, Malaysia. In China, 35 Stylotermes spp. were described between Stylotermes, as the type genus of the new Stylotermitinae subfamily 1963 and 1993 from eight provinces: Fujian [no. -

Termites (Isoptera) in the Azores: an Overview of the Four Invasive Species Currently Present in the Archipelago
Arquipelago - Life and Marine Sciences ISSN: 0873-4704 Termites (Isoptera) in the Azores: an overview of the four invasive species currently present in the archipelago MARIA TERESA FERREIRA ET AL. Ferreira, M.T., P.A.V. Borges, L. Nunes, T.G. Myles, O. Guerreiro & R.H. Schef- frahn 2013. Termites (Isoptera) in the Azores: an overview of the four invasive species currently present in the archipelago. Arquipelago. Life and Marine Sciences 30: 39-55. In this contribution we summarize the current status of the known termites of the Azores (North Atlantic; 37-40° N, 25-31° W). Since 2000, four species of termites have been iden- tified in the Azorean archipelago. These are spreading throughout the islands and becoming common structural and agricultural pests. Two termites of the Kalotermitidae family, Cryp- totermes brevis (Walker) and Kalotermes flavicollis (Fabricius) are found on six and three of the islands, respectively. The other two species, the subterranean termites Reticulitermes grassei Clemént and R. flavipes (Kollar) of the Rhinotermitidae family are found only in confined areas of the cities of Horta (Faial) and Praia da Vitória (Terceira) respectively. Due to its location and weather conditions the Azorean archipelago is vulnerable to coloni- zation by invasive species. The fact that there are four different species of termites in the Azores, all of them considered pests, is a matter of concern. Here we present a comparative description of these species, their known distribution in the archipelago, which control measures are being used against them, and what can be done in the future to eradicate and control these pests in the Azores.