The Plasticity and Developmental Potential of Termites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Symbiotic Cellulolytic Bacteria from the Gut of the Subterranean Termite Psammotermes Hypostoma Desneux and Their Role in Cellulose Digestion Huda R
Ali et al. AMB Expr (2019) 9:111 https://doi.org/10.1186/s13568-019-0830-5 ORIGINAL ARTICLE Open Access Symbiotic cellulolytic bacteria from the gut of the subterranean termite Psammotermes hypostoma Desneux and their role in cellulose digestion Huda R. K. Ali1*, Nada F. Hemeda2 and Yasser F. Abdelaliem3 Abstract The subterranean termite Psammotermes hypostoma Desneux is considered as an important pest that could cause severe damage to buildings, furniture, silos of grain and crops or any material containing cellulose. This species of termites is widespread in Egypt and Africa. The lower termite’s ability to digest cellulose depends on the association of symbiotic organisms gut that digest cellulose (fagellates and bacteria). In this study, 33 diferent bacterial isolates were obtained from the gut of the termite P. hypostoma which were collected using cellulose traps. Strains were grown on carboxymethylcellulose (CMC) as a sole source of carbon. Cellulolytic strains were isolated in two diferent cellulose medium (mineral salt medium containing carboxymethylcellulose as the sole carbon source and agar cel- lulose medium). Five isolates showed signifcant cellulolytic activity identifed by a Congo red assay which gives clear zone. Based on biochemical tests and sequencing of 16s rRNA genes these isolates were identifed as Paenibacillus lactis, Lysinibacillus macrolides, Stenotrophomonas maltophilia, Lysinibacillus fusiformis and Bacillus cereus, that depos- ited in GenBank with accession numbers MG991563, MG991564, MG991565, MG991566 and MG991567, respectively. Keywords: Termite, Psammotermes hypostoma, Symbiosis, Cellulose degrading bacteria, 16S rRNA gene, Phylogenetic analysis Introduction economic damage than food combined with fre (Eggle- Termites are social insects occurring in tropical, subtropical, ton 2011). -

Ant‐Termite Interactions
Biol. Rev. (2020), 95, pp. 555–572. 555 doi: 10.1111/brv.12577 Ant-termite interactions: an important but under-explored ecological linkage Jiri Tuma1,2,3* , Paul Eggleton4 and Tom M. Fayle1,5 1Biology Centre of the Czech Academy of Sciences, Institute of Entomology, Ceske Budejovice, Czech Republic 2Biology Centre of the Czech Academy of Sciences, Institute of Soil Biology, Ceske Budejovice, Czech Republic 3Faculty of Science, University of South Bohemia, Ceske Budejovice, Czech Republic 4Life Sciences Department, Natural History Museum, London, UK 5Institute for Tropical Biology and Conservation, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia ABSTRACT Animal interactions play an important role in understanding ecological processes. The nature and intensity of these inter- actions can shape the impacts of organisms on their environment. Because ants and termites, with their high biomass and range of ecological functions, have considerable effects on their environment, the interaction between them is important for ecosystem processes. Although the manner in which ants and termites interact is becoming increasingly well studied, there has been no synthesis to date of the available literature. Here we review and synthesise all existing literature on ant– termite interactions. We infer that ant predation on termites is the most important, most widespread, and most studied type of interaction. Predatory ant species can regulate termite populations and subsequently slow down the decomposi- tion of wood, litter and soil organic matter. As a consequence they also affect plant growth and distribution, nutrient cycling and nutrient availability. Although some ant species are specialised termite predators, there is probably a high level of opportunistic predation by generalist ant species, and hence their impact on ecosystem processes that termites are known to provide varies at the species level. -

Isoptera) in New Guinea 55 Doi: 10.3897/Zookeys.148.1826 Research Article Launched to Accelerate Biodiversity Research
A peer-reviewed open-access journal ZooKeys 148: 55–103Revision (2011) of the termite family Rhinotermitidae (Isoptera) in New Guinea 55 doi: 10.3897/zookeys.148.1826 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research Revision of the termite family Rhinotermitidae (Isoptera) in New Guinea Thomas Bourguignon1,2,†, Yves Roisin1,‡ 1 Evolutionary Biology and Ecology, CP 160/12, Université Libre de Bruxelles (ULB), Avenue F.D. Roosevelt 50, B-1050 Brussels, Belgium 2 Present address: Graduate School of Environmental Science, Hokkaido Uni- versity, Sapporo 060–0810, Japan † urn:lsid:zoobank.org:author:E269AB62-AC42-4CE9-8E8B-198459078781 ‡ urn:lsid:zoobank.org:author:73DD15F4-6D52-43CD-8E1A-08AB8DDB15FC Corresponding author: Yves Roisin ([email protected]) Academic editor: M. Engel | Received 19 July 2011 | Accepted 28 September 2011 | Published 21 November 2011 urn:lsid:zoobank.org:pub:27B381D6-96F5-482D-B82C-2DFA98DA6814 Citation: Bourguignon T, Roisin Y (2011) Revision of the termite family Rhinotermitidae (Isoptera) in New Guinea. In: Engel MS (Ed) Contributions Celebrating Kumar Krishna. ZooKeys 148: 55–103. doi: 10.3897/zookeys.148.1826 Abstract Recently, we completed a revision of the Termitidae from New Guinea and neighboring islands, record- ing a total of 45 species. Here, we revise a second family, the Rhinotermitidae, to progress towards a full picture of the termite diversity in New Guinea. Altogether, 6 genera and 15 species are recorded, among which two species, Coptotermes gambrinus and Parrhinotermes barbatus, are new to science. The genus Heterotermes is reported from New Guinea for the first time, with two species restricted to the southern part of the island. -

The Termite by Ogden Nash
Biological studies on two European termite species: establishment risk in the UK Laetitia Virginie Laine B.Sc. M.Sc. D.I.C. A thesis submitted for the degree of Doctor of Philosophy of the University of London November 2002 Department of Biological Sciences, Imperial College, Silwood Park, Ascot, SL5 7PY, Berkshire Abstract The discovery of an accidental introduction of termites into Devon in 1994 generated great interest as termites were previously thought to be unable to establish in the UK due to unfavourable climatic conditions. Information about the species present in Devon, Reticulitermes grassei, was found to be lacking and the present study was undertaken to determine the importance of various abiotic and biotic factors in establishment of this species. The factors included in the study were the minimum termite number for establishment, the consumption of wood and its effect on survival and temperature and soil type. A review of the literature was also conducted, detailing the problems with the taxonomy of this termite genus, their present distribution pattern and the life cycle of Reticulitermes species. Two populations of both R. grassei and R. santonensis were studied. The effect of the minimum termite number was found to be significant in both laboratory and field conditions. However, survival decreased in the laboratory and increased in the field with increased number of termites. Consumption experiments were performed using blocks of Scots pine, beech and oak. In most cases termite populations were found to consume and survive best on oak. Consumption was also tested on live seedlings but these results were inconclusive. Survival was observed to increase with increased temperature. -
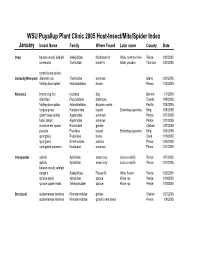
Host Insect List 2005
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

Diversity of Ants and Termites of the Botanical Garden of the University of Lomé, Togo
insects Article Diversity of Ants and Termites of the Botanical Garden of the University of Lomé, Togo Boris Dodji Kasseney 1,* , Titati Bassouo N’tie 1, Yaovi Nuto 1, Dekoninck Wouter 2 , Kolo Yeo 3 and Isabelle Adolé Glitho 1 1 Laboratoire d’Entomologie Appliquée, Département de Zoologie et de Biologie Animale, Université de Lomé, 01 BP 1515, Lomé 01, Lome 151, Togo 2 RBINS Scientific Service Heritage/O.D. Taxonomy and Phylogeny, Curator Entomology Collections, Vautierstraat 29, 1000 Brussels, Belgium 3 Université Nangui Abrogoua, 02 BP 801 Abidjan 02, Abidjan, Côte d’Ivoire * Correspondence: [email protected]; Tel.: +228-906-156-74 Received: 6 June 2019; Accepted: 22 July 2019; Published: 23 July 2019 Abstract: Ants and termites are used as bioindicators in many ecosystems. Little knowledge is available about them in Togo, especially ants. This study aimed to find out how ants and termites could be used to assess the restoration of former agricultural land. These insect groups were sampled within six transects of 50 2 m2 (using pitfall traps, monoliths, baits for ants and hand sampling for × termites) in two consecutive habitats: open area (grassland) and covered area (an artificial forest). Seventeen termite species and 43 ant species were collected. Seven ant species were specific to the covered area against four for the open area, while four unshared species of termite were found in the open area against three in the covered area. The presence of unshared species was linked to vegetation, as Trinervitermes (Holmgren, 1912), a grass feeding termite, was solely found in open area. Also, for some ant species like Cataulacus traegaordhi (Santschi, 1914), Crematogaster (Lund, 1831) species, Oecophylla longinoda (Latreille, 1802) and Tetraponera mocquerysi (Brown, 1960), all arboreal species, vegetation was a determining factor for their presence. -

Taxonomy, Biogeography, and Notes on Termites (Isoptera: Kalotermitidae, Rhinotermitidae, Termitidae) of the Bahamas and Turks and Caicos Islands
SYSTEMATICS Taxonomy, Biogeography, and Notes on Termites (Isoptera: Kalotermitidae, Rhinotermitidae, Termitidae) of the Bahamas and Turks and Caicos Islands RUDOLF H. SCHEFFRAHN,1 JAN KRˇ ECˇ EK,1 JAMES A. CHASE,2 BOUDANATH MAHARAJH,1 3 AND JOHN R. MANGOLD Ann. Entomol. Soc. Am. 99(3): 463Ð486 (2006) ABSTRACT Termite surveys of 33 islands of the Bahamas and Turks and Caicos (BATC) archipelago yielded 3,533 colony samples from 593 sites. Twenty-seven species from three families and 12 genera were recorded as follows: Cryptotermes brevis (Walker), Cr. cavifrons Banks, Cr. cymatofrons Schef- Downloaded from frahn and Krˇecˇek, Cr. bracketti n. sp., Incisitermes bequaerti (Snyder), I. incisus (Silvestri), I. milleri (Emerson), I. rhyzophorae Herna´ndez, I. schwarzi (Banks), I. snyderi (Light), Neotermes castaneus (Burmeister), Ne. jouteli (Banks), Ne. luykxi Nickle and Collins, Ne. mona Banks, Procryptotermes corniceps (Snyder), and Pr. hesperus Scheffrahn and Krˇecˇek (Kalotermitidae); Coptotermes gestroi Wasmann, Heterotermes cardini (Snyder), H. sp., Prorhinotermes simplex Hagen, and Reticulitermes flavipes Koller (Rhinotermitidae); and Anoplotermes bahamensis n. sp., A. inopinatus n. sp., Nasuti- termes corniger (Motschulsky), Na. rippertii Rambur, Parvitermes brooksi (Snyder), and Termes http://aesa.oxfordjournals.org/ hispaniolae Banks (Termitidae). Of these species, three species are known only from the Bahamas, whereas 22 have larger regional indigenous ranges that include Cuba, Florida, or Hispaniola and beyond. Recent exotic immigrations for two of the regional indigenous species cannot be excluded. Three species are nonindigenous pests of known recent immigration. IdentiÞcation keys based on the soldier (or soldierless worker) and the winged imago are provided along with species distributions by island. Cr. bracketti, known only from San Salvador Island, Bahamas, is described from the soldier and imago. -

Glossary - Zoology - Intro
1 Glossary - Zoology - Intro Abdomen: Posterior part of an arthropoda’ body; in vertebrates: abdomen between thorax and pelvic girdle. Acoelous: See coelom. Amixia: A restriction that prevents general intercrossing in a species leading to inbreeding. Anabiosis: Resuscitation after apparent death. Archenteron: See coelom. Aulotomy: Capacity of separating a limb; followed by regeneration; used also for asexual reproduction; see also fissipary (in echinodermata and platyhelminthes). Basal Lamina: Basal plate of developing neural tube; the noncellular, collagenous layer that separates an epithelium from an underlying layer of tissue; also basal membrane. Benthic: Organisms that live on ocean bottoms. Blastocoel: See coelom. Blastula: Stage of embryonic development, at / near the end of cleavage, preceding gastrulation; generally consisting of a hollow ball of cells (coeloblastula); if no blastoceol is present it is termed stereoblastulae (arise from isolecithal and moderately telolecithal ova); in meroblastic cleavage (only the upper part of the zygote is divided), the blastula consists of a disc of cells lying on top of the yolk mass; the blastocoel is reduced to the space separating the cells from the yolk mass. Blastoporus: The opening into the archenteron (the primitive gastric cavity of the gastrula = gastrocoel) developed by the invagination of the blastula = protostoma. Cephalisation: (Gk. kephale, little head) A type of animal body plan or organization in which one end contains a nerve-rich region and functions as a head. Cilium: (pl. cilia, long eyelash) A short, centriole-based, hairlike organelle: Rows of cilia propel certain protista. Cilia also aid the movement of substances across epithelial surfaces of animal cells. Cleavage: The zygote undergoes a series of rapid, synchronous mitotic divisions; results in a ball of many cells. -

Body-Enlarging Effect of Royal Jelly in a Non-Holometabolous Insect Species, Gryllus Bimaculatus
© 2016. Published by The Company of Biologists Ltd | Biology Open (2016) 5, 770-776 doi:10.1242/bio.019190 RESEARCH ARTICLE Body-enlarging effect of royal jelly in a non-holometabolous insect species, Gryllus bimaculatus Atsushi Miyashita, Hayato Kizaki, Kazuhisa Sekimizu and Chikara Kaito* ABSTRACT (Conlon and Raff, 1999; Otto, 2007). These studies have provided Honeybee royal jelly is reported to have body-enlarging effects in significant insight into the principles of size regulation of living holometabolous insects such as the honeybee, fly and silkmoth, but organisms, although recent concerns over genetically modified its effect in non-holometabolous insect species has not yet been organisms have led researchers to evaluate other types of strategies examined. The present study confirmed the body-enlarging effect in to enlarge animals for industrial purposes. silkmoths fed an artificial diet instead of mulberry leaves used in the As a non-genetic size manipulation, oral ingestion of royal jelly previous literature. Administration of honeybee royal jelly to silkmoth by larvae of the honeybee, Apis mellifera, a holometabolous from early larval stage increased the size of female pupae and hymenopteran insect, induces queen differentiation, leading to adult moths, but not larvae (at the late larval stage) or male pupae. enlarged bodies. Royal jelly contains 12-15% protein, 10-16% We further examined the body-enlarging effect of royal jelly in a sugar, 3-6% lipids (percentages are wet-weight basis), vitamins, non-holometabolous species, the two-spotted cricket Gryllus salts, and free amino acids (Buttstedt et al., 2014). Royal jelly bimaculatus, which belongs to the evolutionarily primitive group contains proteins, named major royal jelly proteins (MRJPs), which Polyneoptera. -

Smithsonian Miscellaneous Collections
Ubr.C-ff. SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 143, NO. 3 SUPPLEMENT TO THE ANNOTATED, SUBJECT-HEADING BIBLIOGRAPHY OF TERMITES 1955 TO I960 By THOMAS E. SNYDER Honorary Research Associate Smithsonian Institution (Publication 4463) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION DECEMBER 29, 1961 SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 143, NO. 3 SUPPLEMENT TO THE ANNOTATED, SUBJECT-HEADING BIBLIOGRAPHY OF TERMITES 1955 TO 1960 By THOMAS E. SNYDER Honorary Research Associate Smithsonian Institution ><%<* Q (Publication 4463) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION DECEMBER 29, 1961 PORT CITY PRESS, INC. BALTIMORE, NID., U. S. A. CONTENTS Pagre Introduction i Acknowledgments i List of subject headings 2 Subject headings 3 List of authors and titles 72 Index 115 m SUPPLEMENT TO THE ANNOTATED, SUBJECT-HEADING BIBLIOGRAPHY OF TERMITES 1955 TO 1960 By THOMAS E. SNYDER Honorary Research Associate Smithsonian Institution INTRODUCTION On September 25, 1956, an "Annotated, Subject-Heading Bibliography of Ter- mites 1350 B.C. to A.D. 1954," by Thomas E. Snyder, was published as volume 130 of the Smithsonian Miscellaneous Collections. A few 1955 papers were included. The present supplement covers publications from 1955 through i960; some 1961, as well as some earlier, overlooked papers, are included. A total of 1,150 references are listed under authors and tides, and 2,597 references are listed under subject headings, the greater number being due to cross references to publications covering more than one subject. New subject headings are Radiation and Toxicology. ACKNOWLEDGMENTS The publication of this bibliography was made possible by a grant from the National Science Foundation, Washington, D.C. -

The Impact of Edge Effect on Termite Community (Blattodea: Isoptera) in Fragments of Brazilian Atlantic Rainforest C
http://dx.doi.org/10.1590/1519-6984.17815 Original Article The impact of edge effect on termite community (Blattodea: Isoptera) in fragments of Brazilian Atlantic Rainforest C. S. Almeidaa,b, P. F. Cristaldob, D. F. Florencioc, E. J. M. Ribeirob, N. G. Cruza,b, E. A. Silvad, D. A. Costae and A. P. A. Araújob* aPrograma de Pós-graduação em Ecologia e Conservação, Universidade Federal de Sergipe – UFS, Av. Marechal Rondon, s/n, Jardim Rosa Elze, CEP 49100-000, São Cristóvão, SE, Brazil bLaboratório de Interações Ecológicas, Departamento de Ecologia, Universidade Federal de Sergipe – UFS, Av. Marechal Rondon, s/n, Jardim Rosa Elze, CEP 49100-000, São Cristóvão, SE, Brazil cDepartamento de Agrotecnologia e Ciências Sociais, Universidade Federal Rural do Semi-Árido – UFERSA, BR 110, Km 47, Bairro Pres. Costa e Silva, CP 137, CEP 59625-900, Mossoró, RN, Brazil dPrograma de Pós-graduação em Ciências Ambientais, Universidade Federal de Rondônia – UNIR, Av. Norte Sul, 7300, Bairro Nova Morada, CEP 76940-000, Rolim de Moura, RO, Brazil eDepartamento de Ciências Biológicas, Universidade do Estado de Mato Grosso – UNEMAT, Rod. MT. 358, Km 07, Jd. Aeroporto, CEP 78300-000, Tangará da Serra, MT, Brazil *e-mail: [email protected] Received: October 29, 2015 – Accepted: April 13, 2016 – Distributed: August 31, 2017 (With 1 figure) Abstract Habitat fragmentation is considered to be one of the biggest threats to tropical ecosystem functioning. In this region, termites perform an important ecological role as decomposers and ecosystem engineers. In the present study, we tested whether termite community is negatively affected by edge effects on three fragments of Brazilian Atlantic Rainforest.