Structure and Embryonic Degradation of Two Native Vitellins in the Cockroach, Periplaneta Americana
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cockroach Marion Copeland
Cockroach Marion Copeland Animal series Cockroach Animal Series editor: Jonathan Burt Already published Crow Boria Sax Tortoise Peter Young Ant Charlotte Sleigh Forthcoming Wolf Falcon Garry Marvin Helen Macdonald Bear Parrot Robert E. Bieder Paul Carter Horse Whale Sarah Wintle Joseph Roman Spider Rat Leslie Dick Jonathan Burt Dog Hare Susan McHugh Simon Carnell Snake Bee Drake Stutesman Claire Preston Oyster Rebecca Stott Cockroach Marion Copeland reaktion books Published by reaktion books ltd 79 Farringdon Road London ec1m 3ju, uk www.reaktionbooks.co.uk First published 2003 Copyright © Marion Copeland All rights reserved No part of this publication may be reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior permission of the publishers. Printed and bound in Hong Kong British Library Cataloguing in Publication Data Copeland, Marion Cockroach. – (Animal) 1. Cockroaches 2. Animals and civilization I. Title 595.7’28 isbn 1 86189 192 x Contents Introduction 7 1 A Living Fossil 15 2 What’s in a Name? 44 3 Fellow Traveller 60 4 In the Mind of Man: Myth, Folklore and the Arts 79 5 Tales from the Underside 107 6 Robo-roach 130 7 The Golden Cockroach 148 Timeline 170 Appendix: ‘La Cucaracha’ 172 References 174 Bibliography 186 Associations 189 Websites 190 Acknowledgements 191 Photo Acknowledgements 193 Index 196 Two types of cockroach, from the first major work of American natural history, published in 1747. Introduction The cockroach could not have scuttled along, almost unchanged, for over three hundred million years – some two hundred and ninety-nine million before man evolved – unless it was doing something right. -
Host Insect List 2005
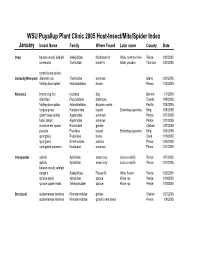
WSU Puyallup Plant Clinic 2005 Host-Insect/Mite/Spider Index January Insect Name Family Where Found Latin name County Date Crop balsam woolly adelgid Adelgididae Nordmann fir Abies nordmannian Pierce 1/31/2005 coneworm Tortricidae noble fir Abies procera Thurston 1/31/2005 comb footed spider, Curiosity/Non-pest Steatoda sp. Theridiidae unknown Island 1/21/2005 folding-door spider Antrodiaetidae house Pierce 1/12/2005 Nuisance brown dog tick Ixodidae dog Benton 1/7/2005 drainflies Psychodidae bathroom Cowlitz 1/28/2005 folding-door spider Antrodiaetidae daycare center Pacific 1/24/2005 fungus gnats Fungivoridae loquat Eriobotrya japonica King 1/24/2005 giant house spider Agelenidae unknown Pierce 1/21/2005 hobo spider Agelenidae unknown Pierce 1/21/2005 moisture ant queen Formicidae garden Clallam 1/27/2005 psocids Psocidae loquat Eriobotrya japonica King 1/24/2005 springtails Poduridae home Clark 1/10/2005 springtails Sminthuridae outside Pierce 1/26/2005 variegated cutworm Noctuidae unknown Pierce 1/21/2005 Ornamental aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 aphids Aphididae sweet bay Laurus nobilis Pierce 1/27/2005 balsam woolly adelgid nymphs Adelgididae Frasier fir Abies fraseri Pierce 1/25/2005 spruce aphid Aphididae spruce Picea sp. Pierce 1/10/2005 spruce spider mites Tetranchyidae spruce Picea sp. Pierce 1/10/2005 Structural subterranean termites Rhinotermitidae garden Clallam 1/27/2005 subterranean termites Rhinotermitidae ground near shed Pierce 1/8/2005 February Insect Name Family Where Found Latin name County -

Isoptera Book Chapter
Isoptera 535 See Also the Following Articles Biodiversity ■ Biogeographical Patterns ■ Cave Insects ■ Introduced Insects Further Reading Carlquist , S. ( 1974 ) . “ Island Biology . ” Columbia University Press , New York and London . Gillespie , R. G. , and Roderick , G. K. ( 2002 ) . Arthropods on islands: Colonization, speciation, and conservation . Annu. Rev. Entomol. 47 , 595 – 632 . Gillespie , R. G. , and Clague , D. A. (eds.) (2009 ) . “ Encyclopedia of Islands. ” University of California Press , Berkeley, CA . Howarth , F. G. , and Mull , W. P. ( 1992 ) . “ Hawaiian Insects and Their Kin . ” University of Hawaii Press , Honolulu, HI . MacArthur , R. H. , and Wilson , E. O. ( 1967 ) . “ The Theory of Island Biogeography . ” Princeton University Press , Princeton, NJ . Wagner , W. L. , and Funk , V. (eds.) ( 1995 ) . “ Hawaiian Biogeography Evolution on a Hot Spot Archipelago. ” Smithsonian Institution Press , Washington, DC . Whittaker , R. J. , and Fern á ndez-Palacios , J. M. ( 2007 ) . “ Island Biogeography: Ecology, Evolution, and Conservation , ” 2nd ed. Oxford University Press , Oxford, U.K . I Isoptera (Termites) Vernard R. Lewis FIGURE 1 Castes for Isoptera. A lower termite group, University of California, Berkeley Reticulitermes, is represented. A large queen is depicted in the center. A king is to the left of the queen. A worker and soldier are he ordinal name Isoptera is of Greek origin and refers to below. (Adapted, with permission from Aventis Environmental the two pairs of straight and very similar wings that termites Science, from The Mallis Handbook of Pest Control, 1997.) Thave as reproductive adults. Termites are small and white to tan or sometimes black. They are sometimes called “ white ants ” and can be confused with true ants (Hymenoptera). -

Sultan Qaboos University Journal for Scientific Research
Agricultural and Marine Sciences, 10(1):33-40 (2005) ©2005 Sultan Qaboos University Identification, Geographical Distribution and Hosts of Subterranean Termites in the United Arab Emirates Arid Ecosystem W. Kaakeh Department of Arid Land Agriculture, College of Food Systems, P. O. Box 17555, United Arab Emirates University, Al-Ain, United Arab Emirates وﻟﯿﺪ ﻛﻌﻚ اﻟﺨﻼﺻﺔ: ﺗﻢ ﺗﻌﺮﻳﻒ ﺳﺘﺔ أﻧﻮاع ﻣﻦ اﻟﻨﻤﻞ اﻷﺑﯿﺾ (اﻷرﺿﺔ) ﺗﺎﺑﻌﺔ إﻟﻰ ﺧﻤﺴﺔ أﺟﻨﺎس وﺛﻼث ﻓﺼﺎﺋﻞ (ھﻮدوjرﻣﯿﺘﯿﺪي Hodotermitidae، راﻳﻨﻮﺗﺮﻣﯿﺘﯿﺪي Rhinotermi،Rhinotermitidaetidae، وﺗﺮﻣﯿﺘﯿﺪي T(Termitidaeermitidae) ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وأﻧﻮاع اﻷرﺿﺔ اﻟﺘﻲ ﺗﻢ ﺗﺴﺠﯿﻠﮭﺎ ھﻲ اﻷرﺿﺔ اﻟﺤﺎﺻﺪة أو اﻷرﺿﺔ اﻟﻼﺷﻮﻛﯿﺔ Anacanthotermes ochraceusochraceus (Burmeister(Burmeister) و Anacanthotermes ubachi (Navas(Navas)، وأرﺿﺔ اﻟﺮﻣﻞ اﻟﺜﻐﺮﻳﺔ Psammotermes hypostomahypostoma (Desneux)، واﻷرﺿﺔ اﻟﺸﻤﻌﯿﺔ اﻟﺼﻐﯿﺮة MicrocerotermesMicrocerotermes diversusdiversus Silvestri))، واﻷرﺿﺔ اﻟﻨﺠﺪﻳﺔ اﻟﺪﻗﯿﻘﺔ Microtermes najdensis (Harris) ، واﻷرﺿﺔ Heterotermes aethiopicus (Sjostedt)، وﺑﺎﺳﺘﺜﻨﺎء اﻟﻨﻮع H. aethiopicus، ﻓﺈﻧﻪ ﺗﻢ ﺗﺴﺠﯿﻞ اﻷﻧﻮاع اﻟﺨﻤﺴﺔ اﻷﺧﺮى ﻟﻠﻤﺮة اﻷوﻟﻰ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة. وﺗﻌﯿﺶ ﻛﻞ اﻷﻧﻮاع ﺗﺤﺖ اﻷرض وﺗﺼﻞ إﻟﻰ ﻣﺼﺎدر اﻟﻐﺬاء اﻟﺨﺸﺒﯿﺔ ﻣﻦ ﺧﻼل أﻧﻈﻤﺔ اﻷﻧﻔﺎق اﻟﻄﯿﻨﯿﺔ. وﻗﺪ وﺟﺪت اﻷرﺿﺔ ﻓﻲ ﻣﻨﺎﻃﻖ ﻣﺨﺘﻠﻔﺔ ﻣﻦ اﻟﺪوﻟﺔ واﻟﺘﻲ ﺗﺘﻤﯿﺰ ﺑﺎﺧﺘﻼف ﻇﺮوﻓﮭﺎ اﻟﻤﻨﺎﺧﯿﺔ وﻏﻄﺎءھﺎ اﻟﻨﺒﺎﺗﻲ وﻧﻮع ﺗﺮﺑﺘﮭﺎ. وﺗﻔﻀﻞ اﻷرﺿﺔ اﻟﺘﻐﺬﻳﺔ ﻋﻠﻰ اﻟﻌﻮاﺋﻞ اﻟﺤﯿﺔ أو اﻟﻤﯿﺘﺔ أو اﻟﻤﺘﻌﻔﻨﺔ، ﺑﺎﻹﺿﺎﻓﺔ إﻟﻰ اﻟﻤﻮاد ﻏﯿﺮ اﻟﺴﯿﻠﯿﻠﻮزﻳﺔ. وﻣﻦ أﻛﺜﺮ أﻧﻮاع اﻷرﺿﺔ ًﺗﻮزﻳﻌﺎ ﻓﻲ اﻹﻣﺎرات اﻟﻌﺮﺑﯿﺔ اﻟﻤﺘﺤﺪة ھﻲ A. ochraceusochraceus وﺗﺘﺒﻌﮭﺎ ﻛﻞ ﻣﻦ P.P. hypostomahypostoma وdiversusوM. diversus. وﻗﺪ اﺧﺘﻠﻒ ﺗﻮزﻳﻊ اﻷﻧﻮاع اﻟﺴﺘﺔ ﺿﻤﻦ -

Blattodea: Hodotermitidae) and Its Role As a Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia
Article Characterization of the 12S rRNA Gene Sequences of the Harvester Termite Anacanthotermes ochraceus (Blattodea: Hodotermitidae) and Its Role as A Bioindicator of Heavy Metal Accumulation Risks in Saudi Arabia Reem Alajmi 1,*, Rewaida Abdel-Gaber 1,2,* and Noura AlOtaibi 3 1 Zoology Department, College of Science, King Saud University, Riyadh 11451, Saudi Arabia 2 Zoology Department, Faculty of Science, Cairo University, Cairo 12613, Egypt 3 Department of Biology, Faculty of Science, Taif University, Taif 21974, Saudi Arabia; [email protected] * Correspondence: [email protected] (R.A.), [email protected] (R.A.-G.) Received: 28 December 2018; Accepted: 3 February 2019; Published: 8 February 2019 Abstract: Termites are social insects of economic importance that have a worldwide distribution. Identifying termite species has traditionally relied on morphometric characters. Recently, several mitochondrial genes have been used as genetic markers to determine the correlation between different species. Heavy metal accumulation causes serious health problems in humans and animals. Being involved in the food chain, insects are used as bioindicators of heavy metals. In the present study, 100 termite individuals of Anacanthotermes ochraceus were collected from two Saudi Arabian localities with different geoclimatic conditions (Riyadh and Taif). These individuals were subjected to morphological identification followed by molecular analysis using mitochondrial 12S rRNA gene sequence, thus confirming the morphological identification of A. ochraceus. Furthermore, a phylogenetic analysis was conducted to determine the genetic relationship between the acquired species and other termite species with sequences previously submitted in the GenBank database. Several heavy metals including Ca, Al, Mg, Zn, Fe, Cu, Mn, Ba, Cr, Co, Be, Ni, V, Pb, Cd, and Mo were measured in both collected termites and soil samples from both study sites. -

RESEARCH ARTICLE a New Species of Cockroach, Periplaneta
Tropical Biomedicine 38(2): 48-52 (2021) https://doi.org/10.47665/tb.38.2.036 RESEARCH ARTICLE A new species of cockroach, Periplaneta gajajimana sp. nov., collected in Gajajima, Kagoshima Prefecture, Japan Komatsu, N.1, Iio, H.2, Ooi, H.K.3* 1Civil International Corporation, 10–14 Kitaueno 1, Taito–ku, Tokyo, 110–0014, Japan 2Foundation for the Protection of Deer in Nara, 160-1 Kasugano-cho, Nara-City, Nara, 630-8212, Japan 3Laboratory of Parasitology, School of Veterinary Medicine, Azabu University, 1-17-710 Fuchinobe, Sagamihara, Kanagawa 252-5201 Japan *Corresponding author: [email protected] ARTICLE HISTORY ABSTRACT Received: 25 January 2021 We described a new species of cockroach, Periplaneta gajajimana sp. nov., which was collected Revised: 2 February 2021 in Gajajima, Kagoshima-gun Toshimamura, Kagoshima Prefecture, Japan, on November 2012. Accepted: 2 February 2021 The new species is characterized by its reddish brown to blackish brown body, smooth Published: 30 April 2021 surface pronotum, well developed compound eyes, dark brown head apex, dark reddish brown front face and small white ocelli connected to the antennal sockets. In male, the tegmen tip reach the abdomen end or are slightly shorter, while in the female, it does not reach the abdominal end and exposes the abdomen beyond the 7th abdominal plate. We confirmed the validity of this new species by breeding the specimens in our laboratory to demonstrate that the features of the progeny were maintained for several generations. For comparison and easy identification of this new species, the key to species identification of the genus Periplaneta that had been reported in Japan to date are also presented. -

The Phylogeny of Termites
Molecular Phylogenetics and Evolution 48 (2008) 615–627 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev The phylogeny of termites (Dictyoptera: Isoptera) based on mitochondrial and nuclear markers: Implications for the evolution of the worker and pseudergate castes, and foraging behaviors Frédéric Legendre a,*, Michael F. Whiting b, Christian Bordereau c, Eliana M. Cancello d, Theodore A. Evans e, Philippe Grandcolas a a Muséum national d’Histoire naturelle, Département Systématique et Évolution, UMR 5202, CNRS, CP 50 (Entomologie), 45 rue Buffon, 75005 Paris, France b Department of Integrative Biology, 693 Widtsoe Building, Brigham Young University, Provo, UT 84602, USA c UMR 5548, Développement—Communication chimique, Université de Bourgogne, 6, Bd Gabriel 21000 Dijon, France d Muzeu de Zoologia da Universidade de São Paulo, Avenida Nazaré 481, 04263-000 São Paulo, SP, Brazil e CSIRO Entomology, Ecosystem Management: Functional Biodiversity, Canberra, Australia article info abstract Article history: A phylogenetic hypothesis of termite relationships was inferred from DNA sequence data. Seven gene Received 31 October 2007 fragments (12S rDNA, 16S rDNA, 18S rDNA, 28S rDNA, cytochrome oxidase I, cytochrome oxidase II Revised 25 March 2008 and cytochrome b) were sequenced for 40 termite exemplars, representing all termite families and 14 Accepted 9 April 2008 outgroups. Termites were found to be monophyletic with Mastotermes darwiniensis (Mastotermitidae) Available online 27 May 2008 as sister group to the remainder of the termites. In this remainder, the family Kalotermitidae was sister group to other families. The families Kalotermitidae, Hodotermitidae and Termitidae were retrieved as Keywords: monophyletic whereas the Termopsidae and Rhinotermitidae appeared paraphyletic. -

Mcabee Fossil Site Assessment
1 McAbee Fossil Site Assessment Final Report July 30, 2007 Revised August 5, 2007 Further revised October 24, 2008 Contract CCLAL08009 by Mark V. H. Wilson, Ph.D. Edmonton, Alberta, Canada Phone 780 435 6501; email [email protected] 2 Table of Contents Executive Summary ..............................................................................................................................................................3 McAbee Fossil Site Assessment ..........................................................................................................................................4 Introduction .......................................................................................................................................................................4 Geological Context ...........................................................................................................................................................8 Claim Use and Impact ....................................................................................................................................................10 Quality, Abundance, and Importance of the Fossils from McAbee ............................................................................11 Sale and Private Use of Fossils from McAbee..............................................................................................................12 Educational Use of Fossils from McAbee.....................................................................................................................13 -

The American Cockroach, Periplaneta Americana Linnaeus, As a Disseminator of Some Salmonella Bacteria
University of Massachusetts Amherst ScholarWorks@UMass Amherst Doctoral Dissertations 1896 - February 2014 1-1-1943 The American cockroach, Periplaneta americana Linnaeus, as a disseminator of some Salmonella bacteria. Arnold Erwin Fischman University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/dissertations_1 Recommended Citation Fischman, Arnold Erwin, "The American cockroach, Periplaneta americana Linnaeus, as a disseminator of some Salmonella bacteria." (1943). Doctoral Dissertations 1896 - February 2014. 5573. https://scholarworks.umass.edu/dissertations_1/5573 This Open Access Dissertation is brought to you for free and open access by ScholarWorks@UMass Amherst. It has been accepted for inclusion in Doctoral Dissertations 1896 - February 2014 by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. 3120bfc. 0230 2b3D b '! HE AMERICAN COCKROACH, ITiRIPEANETA AMERICANA LINNAEUS AS A DISSEMINATOR OF SOME SAl_.MONEL.LA BACTERIA — 111 F1SCHMAN - 1843 MORR LD 3234 ! M267 11943 F529 THK A&SBiCAjf cockroach, mSSSABk NKEJBUk ummxjs AS A PISSE’CHATOR CHP SO* RkUKMSUL BACTERIA Arnold Erwin Plachaan Thesis subaittetf in partial fulfill wont of the requirements for the degree of Doctor of Riiloeophy Shseaohuaetta State College May, 1943 TABLE OP COSmtrs Jhge X. INTKGfUCTIGN .... 1 1. Origin, I-iatribution and Abundance of the Cockroach 1 2. Importance of the Cockroach •••••••••••••• 2 II. RETIES OP LITERATURE .. 6 1. Morphology of the Cockroach •••••••••••••• 7 2. Pevelopment of the Cockroach •••••••••••.. 7 3* Biology of the American Cockroach, Perl- nlcrmt* aaarloana Linnaeus •••••••••••• 8 4* Control .. 10 5. Bacteria and the Cockroach .. 12 6. Virus and the Cockroach 25 7. FUngi and the Cockroach ••»•••••••••••.••• 25 8. -

A Dichotomous Key for the Identification of the Cockroach Fauna (Insecta: Blattaria) of Florida
Species Identification - Cockroaches of Florida 1 A Dichotomous Key for the Identification of the Cockroach fauna (Insecta: Blattaria) of Florida Insect Classification Exercise Department of Entomology and Nematology University of Florida, Gainesville 32611 Abstract: Students used available literature and specimens to produce a dichotomous key to species of cockroaches recorded from Florida. This exercise introduced students to techniques used in studying a group of insects, in this case Blattaria, to produce a regional species key. Producing a guide to a group of insects as a class exercise has proven useful both as a teaching tool and as a method to generate information for the public. Key Words: Blattaria, Florida, Blatta, Eurycotis, Periplaneta, Arenivaga, Compsodes, Holocompsa, Myrmecoblatta, Blatella, Cariblatta, Chorisoneura, Euthlastoblatta, Ischnoptera,Latiblatta, Neoblatella, Parcoblatta, Plectoptera, Supella, Symploce,Blaberus, Epilampra, Hemiblabera, Nauphoeta, Panchlora, Phoetalia, Pycnoscelis, Rhyparobia, distributions, systematics, education, teaching, techniques. Identification of cockroaches is limited here to adults. A major source of confusion is the recogni- tion of adults from nymphs (Figs. 1, 2). There are subjective differences, as well as morphological differences. Immature cockroaches are known as nymphs. Nymphs closely resemble adults except nymphs are generally smaller and lack wings and genital openings or copulatory appendages at the tip of their abdomen. Many species, however, have wingless adult females. Nymphs of these may be recognized by their shorter, relatively broad cerci and lack of external genitalia. Male cockroaches possess styli in addition to paired cerci. Styli arise from the subgenital plate and are generally con- spicuous, but may also be reduced in some species. Styli are absent in adult females and nymphs. -

Very Similar to 'M.' Limai, Except for the Distinctly Larger Size, and Thus
Insects of the Crato Formation 249 Comment: very similar to ‘M.’ limai, except for the distinctly larger size, and thus probably belonging to the same new genus. It has not been possible to determine whether this new taxon is conspecific with the undescribed new genus and species mentioned by Vrˇsansk´y (2004) or instead represents a third blattellid taxon from Crato. Blattidae The presence of the Blattidae in the Crato Formation was first noted by Mendes (1993), who recognized that Mesoblattinopsis schneideri Pinto, 1989 was a blattid. Mesoblattinopsis schneideri Pinto, 1989 Comment: two further new species of Mesoblattinopsis are reported by Mendes (1997b). Family incertae sedis Unnamed new genus and species B Material: three specimens with nos SMNS 66321, SMNS 66308 (Figure 11.23h) and SMNS 66309. Diagnosis: body length about 8.7–9.5 mm; shape of body longish oval; antennae about as long as body; pronotum much broader than head (width 3.0–3.7 mm, thus 180–195% of head width), posteriorly broader than anteriorly, but with narrower lateral lobes than the new blattellid species mentioned above; forewing venation unknown, but with a broad costal margin; cerci with about 10 segments. 11.9 Isoptera: termites G¨unter Bechly There are about 2,800 Recent and about 130 fossil termite species, usually classified in seven families (see below). Termites are relatively small insects with a body length that is usually much less than 3 cm. They are soft-bodied and often called white ants because they are small colonial insects of more or less whitish color. They are, however, completely unrelated to ants, but closely related to cockroaches and mantids. -

Order Blattodea*
Zootaxa 3703 (1): 046–048 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2013 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3703.1.10 http://zoobank.org/urn:lsid:zoobank.org:pub:72196B26-433A-4816-90B3-9EC15495E1B4 Order Blattodea* GEORGE BECCALONI1 & PAUL EGGLETON2 1Curator of orthopteroid insects, Life Sciences Department, Terrestrial Invertebrates Division, The Natural History Museum, London SW7 5BD, UK; email: [email protected] 2Merit Researcher, Life Sciences Department, Terrestrial Invertebrates Division, The Natural History Museum, London SW7 5BD, UK * In: Zhang, Z.-Q. (Ed.) Animal Biodiversity: An Outline of Higher-level Classification and Survey of Taxonomic Richness (Addenda 2013). Zootaxa, 3703, 1–82. Abstract The Blattodea comprise the termites (epifamily Termitoidae only) and the cockroaches (all other taxa). 7570 living species of Blattodea are currently recognised, of which 2929 are termites (Krishna et al. 2013) and 4641 are cockroaches (Beccaloni 2007) . Key words: Blattodea, cockroaches, termites, classification, diversity Introduction The Blattodea comprise the termites (epifamily Termitoidae only) and the cockroaches (all other taxa). Beccaloni and Eggleton (2011) recognized 7314 extant named species of Blattodea, including 2692 termites and 4622 cockroaches and. In this update, 7570 living species of Blattodea are currently recognised, of which 2929 are termites (Krishna et al. 2013) and 4641 are cockroaches (Beccaloni 2007) . Inward, Beccaloni & Eggleton (2007) and subsequent phylogenetic studies (Legendre et al. 2008; Ware et al. 2008; Cameron et al. 2012; Djernaes et al. 2012; Xiao et al. 2012) have confirmed that the termites and the cockroach family Cryptocercidae are sister groups and that this clade is nested within the Blattodea.