Hexachlorobenzene - Sources, Environmental Fate and Risk Characterisation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Daily Eastern News: August 26, 2004 Eastern Illinois University
Eastern Illinois University The Keep August 2004 8-26-2004 Daily Eastern News: August 26, 2004 Eastern Illinois University Follow this and additional works at: http://thekeep.eiu.edu/den_2004_aug Recommended Citation Eastern Illinois University, "Daily Eastern News: August 26, 2004" (2004). August. 9. http://thekeep.eiu.edu/den_2004_aug/9 This is brought to you for free and open access by the 2004 at The Keep. It has been accepted for inclusion in August by an authorized administrator of The Keep. For more information, please contact [email protected]. ''Tell the truth 111ul don't be afraiJ. " 1IE VOCE + IHkilc .. fn1ll•111: page 1 B THE DAILY TllURSDIY AUGUST 26 2004 thedailyeastemnews.com Eastern Illinois University, Charkston f"trefighters work around a vehicle in the driveway, which also reuived damage from the tire. The cause of the fire, which started in the far west room of the house, is under investigation. STEPHEN H AAS'THEDAILYEASTtRN M WS II II 1r I Home of track members totaled in afternoon blaze BY JULIA BoURQUf and Eric Buhot and juniors Nathan Pepper and CAMPUS EDITOR Daniel St:raekdjahn live in the "Arby's house," located near the Arby's on Lincoln Avenue. The The house of Eascem traek and cross councry house bas been rhe crack and cross councry team members caughc fire Wednesday arrer house for three yC:irs, and Atkins was a founding noon, leaving behind severe member. damage and 6vc Ea.stem ath Sinc.c Carmen Hall's fire, uni leces withour a home. More on the Web versity officials have developed The fire srarced in rhe fu See more photos at: an emergency alrernative plan, wesuoom ofthe house at 1515 ''"'"' .1hedai/ye.i.<ternf')('W$. -

Herbicide Mode of Action Table High Resistance Risk
Herbicide Mode of Action Table High resistance risk Chemical family Active constituent (first registered trade name) GROUP 1 Inhibition of acetyl co-enzyme A carboxylase (ACC’ase inhibitors) clodinafop (Topik®), cyhalofop (Agixa®*, Barnstorm®), diclofop (Cheetah® Gold* Decision®*, Hoegrass®), Aryloxyphenoxy- fenoxaprop (Cheetah®, Gold*, Wildcat®), fluazifop propionates (FOPs) (Fusilade®), haloxyfop (Verdict®), propaquizafop (Shogun®), quizalofop (Targa®) Cyclohexanediones (DIMs) butroxydim (Factor®*), clethodim (Select®), profoxydim (Aura®), sethoxydim (Cheetah® Gold*, Decision®*), tralkoxydim (Achieve®) Phenylpyrazoles (DENs) pinoxaden (Axial®) GROUP 2 Inhibition of acetolactate synthase (ALS inhibitors), acetohydroxyacid synthase (AHAS) Imidazolinones (IMIs) imazamox (Intervix®*, Raptor®), imazapic (Bobcat I-Maxx®*, Flame®, Midas®*, OnDuty®*), imazapyr (Arsenal Xpress®*, Intervix®*, Lightning®*, Midas®* OnDuty®*), imazethapyr (Lightning®*, Spinnaker®) Pyrimidinyl–thio- bispyribac (Nominee®), pyrithiobac (Staple®) benzoates Sulfonylureas (SUs) azimsulfuron (Gulliver®), bensulfuron (Londax®), chlorsulfuron (Glean®), ethoxysulfuron (Hero®), foramsulfuron (Tribute®), halosulfuron (Sempra®), iodosulfuron (Hussar®), mesosulfuron (Atlantis®), metsulfuron (Ally®, Harmony®* M, Stinger®*, Trounce®*, Ultimate Brushweed®* Herbicide), prosulfuron (Casper®*), rimsulfuron (Titus®), sulfometuron (Oust®, Eucmix Pre Plant®*, Trimac Plus®*), sulfosulfuron (Monza®), thifensulfuron (Harmony®* M), triasulfuron (Logran®, Logran® B-Power®*), tribenuron (Express®), -

Exposure to Herbicides in House Dust and Risk of Childhood Acute Lymphoblastic Leukemia
Journal of Exposure Science and Environmental Epidemiology (2013) 23, 363–370 & 2013 Nature America, Inc. All rights reserved 1559-0631/13 www.nature.com/jes ORIGINAL ARTICLE Exposure to herbicides in house dust and risk of childhood acute lymphoblastic leukemia Catherine Metayer1, Joanne S. Colt2, Patricia A. Buffler1, Helen D. Reed3, Steve Selvin1, Vonda Crouse4 and Mary H. Ward2 We examine the association between exposure to herbicides and childhood acute lymphoblastic leukemia (ALL). Dust samples were collected from homes of 269 ALL cases and 333 healthy controls (o8 years of age at diagnosis/reference date and residing in same home since diagnosis/reference date) in California, using a high-volume surface sampler or household vacuum bags. Amounts of agricultural or professional herbicides (alachlor, metolachlor, bromoxynil, bromoxynil octanoate, pebulate, butylate, prometryn, simazine, ethalfluralin, and pendimethalin) and residential herbicides (cyanazine, trifluralin, 2-methyl-4- chlorophenoxyacetic acid (MCPA), mecoprop, 2,4-dichlorophenoxyacetic acid (2,4-D), chlorthal, and dicamba) were measured. Odds ratios (OR) and 95% confidence intervals (CI) were estimated by logistic regression. Models included the herbicide of interest, age, sex, race/ethnicity, household income, year and season of dust sampling, neighborhood type, and residence type. The risk of childhood ALL was associated with dust levels of chlorthal; compared to homes with no detections, ORs for the first, second, and third tertiles were 1.49 (95% CI: 0.82–2.72), 1.49 (95% CI: 0.83–2.67), and 1.57 (95% CI: 0.90–2.73), respectively (P-value for linear trend ¼ 0.05). The magnitude of this association appeared to be higher in the presence of alachlor. -

Iggy & the Stooges
3/29/2011 Iggy & the Stooges - "Raw Power Live:… Share Report Abuse Next Blog» Create Blog Sign In Ads by Google Band Iggy Pop Video DVD Disc Disc Jockey Music SATURDAY, MARCH 05, 2011 HELP PUT CHICAGO'S EMPIRES ON THE COVER OF ROLLING STONE Iggy & the Stooges - "Raw Power Live: In the Hands of the Fans" CD Review (MVD) RollingStone Do you wanna be a Rock & Roll Star? Presented by Garnier Fructis On Friday, Sept. 3rd last year, Iggy & the Stooges (featuring James Williamson on guitar) performed Help put 197 3's Raw Power in its entirety at All Empires Tomorrow's Parties New York. A live document of on the cover of this show, Raw Power Live: In the Hands of the Fans , is coming out next month as a limited edition 180 gram vinyl release for Record Store Day 2011. "Getting this top-notch performance of the entire Raw Power album by The Stooges realized a life long dream, " said Iggy Pop " This shit really sizzles RATE THIS and we are so obviously a crack band in a class 1 of our own. " 2 3 4 5 BrooklynRocks Concert Calendar Today Tuesday, March 29 Thursday, March 31 8:00pm Bridge to Japan (Benefit Concert) Saturday, April 2 8:00pm Tommy Rodgers (BTBAM) Saturday, April 9 10:00pm Surf City / Bardo Pond Monday, April 11 9:00pm The Builders and The Butchers Tuesday, April 12 9:00pm Surf City Thursday, April 28 Events show n in time zone: Eastern Time CONTACT INFO. Billboard Magazine had the following to say about The Stooges' performance: " [a]s the pretty clear M IKE main attraction, Iggy and the Stooges granted a predictably explosive show. -
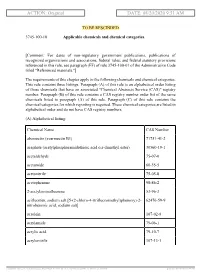
ACTION: Original DATE: 08/20/2020 9:51 AM
ACTION: Original DATE: 08/20/2020 9:51 AM TO BE RESCINDED 3745-100-10 Applicable chemicals and chemical categories. [Comment: For dates of non-regulatory government publications, publications of recognized organizations and associations, federal rules, and federal statutory provisions referenced in this rule, see paragraph (FF) of rule 3745-100-01 of the Administrative Code titled "Referenced materials."] The requirements of this chapter apply to the following chemicals and chemical categories. This rule contains three listings. Paragraph (A) of this rule is an alphabetical order listing of those chemicals that have an associated "Chemical Abstracts Service (CAS)" registry number. Paragraph (B) of this rule contains a CAS registry number order list of the same chemicals listed in paragraph (A) of this rule. Paragraph (C) of this rule contains the chemical categories for which reporting is required. These chemical categories are listed in alphabetical order and do not have CAS registry numbers. (A) Alphabetical listing: Chemical Name CAS Number abamectin (avermectin B1) 71751-41-2 acephate (acetylphosphoramidothioic acid o,s-dimethyl ester) 30560-19-1 acetaldehyde 75-07-0 acetamide 60-35-5 acetonitrile 75-05-8 acetophenone 98-86-2 2-acetylaminofluorene 53-96-3 acifluorfen, sodium salt [5-(2-chloro-4-(trifluoromethyl)phenoxy)-2- 62476-59-9 nitrobenzoic acid, sodium salt] acrolein 107-02-8 acrylamide 79-06-1 acrylic acid 79-10-7 acrylonitrile 107-13-1 [ stylesheet: rule.xsl 2.14, authoring tool: RAS XMetaL R2_0F1, (dv: 0, p: 185720, pa: -

OCTOBER 19, 1934 5 Cents the Copy
l Mr. Howa:rct M. Chapin R. I. Historical Society 68 Waterman st. Providence, R. I. THE HOME NEV/SPAPER OF RHODE ISLAND AND SOUTHERN MASSACHUSETTS Vol. X, No. 7 PROVIDENCE, R. I., FRIDAY, OCTOBER 19, 1934 5 Cents the Copy Propose Central Council Unique Dedication GERMANS MAD, for Exchange of Data, Series Tonight at SAYS DR.BUTLER Information Plans Temple Emanu-El AT COLLEGE RITE Trend to East Side - I SCHENECTA DY. K. y_ (JT.-\)-T he S5.000 extens ion to the pres· Everyone is ,velcomed to Dr. :\'ich olas 1\l urra y Butler, p res i- ent g y mnasium building and t h e for Unveiling of Stained d ent of Columbia Unive rs itv. s aid to- 11:1 a tion of an_ educational and recrea day that the people of Ger~any haYe t,onal council and the extension of Glass Windows gone "sta rk. r a ,·ing mad." the ~enter pro¥'ram to the South . IPrond ence s ection of the city were .-\ Service which promis es to be . Speakm_g at cerem~m1es marking the highlig hts of the s urvey m ade unique in the history of th.is J ewish inauguration of Dr. Dixon Rya.n Fox br the J ewish Welfare Board of community will be held at Temple as the 12th president of Union Col- New York and presented by Ema.n u·El tonight. when 13 stained· l~ge, Dr. Butler de~lared that "no Louis Kraft, director of J ewish Cen. RABBI LE\'! A . OLAN REV. -

AP-42, CH 9.2.2: Pesticide Application
9.2.2PesticideApplication 9.2.2.1General1-2 Pesticidesaresubstancesormixturesusedtocontrolplantandanimallifeforthepurposesof increasingandimprovingagriculturalproduction,protectingpublichealthfrompest-bornediseaseand discomfort,reducingpropertydamagecausedbypests,andimprovingtheaestheticqualityofoutdoor orindoorsurroundings.Pesticidesareusedwidelyinagriculture,byhomeowners,byindustry,andby governmentagencies.Thelargestusageofchemicalswithpesticidalactivity,byweightof"active ingredient"(AI),isinagriculture.Agriculturalpesticidesareusedforcost-effectivecontrolofweeds, insects,mites,fungi,nematodes,andotherthreatstotheyield,quality,orsafetyoffood.Theannual U.S.usageofpesticideAIs(i.e.,insecticides,herbicides,andfungicides)isover800millionpounds. AiremissionsfrompesticideusearisebecauseofthevolatilenatureofmanyAIs,solvents, andotheradditivesusedinformulations,andofthedustynatureofsomeformulations.Mostmodern pesticidesareorganiccompounds.EmissionscanresultdirectlyduringapplicationorastheAIor solventvolatilizesovertimefromsoilandvegetation.Thisdiscussionwillfocusonemissionfactors forvolatilization.Thereareinsufficientdataavailableonparticulateemissionstopermitemission factordevelopment. 9.2.2.2ProcessDescription3-6 ApplicationMethods- Pesticideapplicationmethodsvaryaccordingtothetargetpestandtothecroporothervalue tobeprotected.Insomecases,thepesticideisapplieddirectlytothepest,andinotherstothehost plant.Instillothers,itisusedonthesoilorinanenclosedairspace.Pesticidemanufacturershave developedvariousformulationsofAIstomeetboththepestcontrolneedsandthepreferred -

Cascade Range
United States bepartment of Agriculture Herbicide Forest Service Pacific Northwest Forest and Range and Conifer Experiment Station Research Paper PNW-292 Options for Reforesting October, 1981 Upper Slopes in the Cascade Range Edward J. Dimock II ROCKY Mof 'NTATN STATION This publication reports research involving pesticides. It does not contain recommendations for their use, nor does it imply that the uses discussed have been registered. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. CAUTION: Pesticides can be injurious to humans, domestic animals, desirable Author plants, and fish or other wildlife -- if they I~ m w ~llmml are not handled or applied properly. Use EDWARD J. DIMOCK II is principal all pesticides selectively and carefully. silviculturist, Forestry Sciences Follow recommended practices for the Laboratory, 3200 Jefferson Way, disposal of surplus pesticides and Corvallis, Oregon 97331. pesticide containers. Abstract Summary Dimock, Edward J. I1. Herbicide and Nine herbicides (asulam, atrazine, Similar survival increases were also conifer options for reforesting upper bromacil, cyanazine, dalapon, consistently achieved with atrazine + slopes in the Cascade Range. Res. glyphosate, hexazinone, pronamide, and dalapon mixtures. Most successful Pap. PNW-292. Portland, OR: U.S. terbacil) were evaluated for control of applications of mixture produced 3-year Department of Agriculture, Forest sedge (Carex spp.) and beargrass survival of 62, 77, and 14 percent for white Service, Pacific Northwest Forest (Xerophyllum tenax [Pursh] Nutt.) to aid pine at the same three localities above. and Range Experiment Station; establishment of newly planted seedlings Survival of other conifers resulting from 1981.14 p. -

Redox Imbalance Caused by Pesticides: a Review of OPENTOX-Related Research
Marjanović Čermak AM, et al. Redox imbalance caused by pesticides Arh Hig Rada Toksikol 2018;69:126-134 126 Review DOI: 10.2478/aiht-2018-69-3105 Redox imbalance caused by pesticides: a review of OPENTOX-related research Ana Marija Marjanović Čermak, Ivan Pavičić, and Davor Želježić Institute for Medical Research and Occupational Health, Zagreb, Croatia [Received in February 2018; Similarity Check in February 2018; Accepted in May 2018] Pesticides are a highly diverse group of compounds and the most important chemical stressors in the environment. Mechanisms that could explain pesticide toxicity are constantly being studied and their interactions at the cellular level are often observed in well-controlled in vitro studies. Several pesticide groups have been found to impair the redox balance in the cell, but the mechanisms leading to oxidative stress for certain pesticides are only partly understood. As our scientific project “Organic pollutants in environment – markers and biomarkers of toxicity (OPENTOX)” is dedicated to studying toxic effects of selected insecticides and herbicides, this review is focused on reporting the knowledge regarding oxidative stress-related phenomena at the cellular level. We wanted to single out the most important facts relevant to the evaluation of our own findings from studies conducted onin vitro cell models. KEY WORDS: antioxidants; apoptosis; glyphosate; in vitro; neonicotinoids; organophosphates; oxidative stress; pyrethroids; reactive oxygen species Over the years, population growth and changes in food (HrZZ), is dedicated to studying the toxic effects of two consumption patterns have challenged agricultural major pesticide classes with three subgroups each: (A) production to meet the demand for food and quality insecticides (organophosphates, neonicotinoids, and standards. -

Hexachlorobenzene UNITED NATIONS
UNITED NATIONS RC UNEP/FAO/RC/CRC.5/10/Add.1 Distr.: General 2 December 2008 United Nations English only Environment Programme Food and Agriculture Organization of the United Nations Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade Chemical Review Committee Fifth meeting Rome, 23–27 March 2009 Item 4 (b) (vii) of the provisional agenda* Listing of chemicals in Annex III to the Rotterdam Convention: review of notifications of final regulatory actions to ban or severely restrict a chemical: hexachlorobenzene Hexachlorobenzene Note by the Secretariat Addendum Supporting documentation provided by Canada The Secretariat has the honour to provide, in the annex to the present note, documentation received from Canada to support its notification of final regulatory action on hexachlorobenzene as an industrial chemical. * UNEP/FAO/RC/CRC.5/1. K0842870 161208 For reasons of economy, this document is printed in a limited number. Delegates are kindly requested to bring their copies to meetings and not to request additional copies. UNEP/FAO/RC/CRC.5/10/Add.1 Annex • Hexachlorobenzene: Priority Substances List Assessment Report, Environment Canada, Health Canada. • Environmental Assessments of Priority Substances Under the Canadian Environmental Protection Act, Guidance Manual, Version 1.0 — March 1997. Environment Canada. • SOR/2005-41; CANADIAN ENVIRONMENTAL PROTECTION ACT, 1999; Prohibition of Certain Toxic Substances Regulations, 2005, Canada Government Gazette. 2 Canadian -

Version, Februari 4Th, 2000 Dutch Target and Intervention
www.esdat.net Esdat Environmental Database Management Software +61 2 9232 8080 Version, februari 4th, 2000 Dutch Target and Intervention Values, 2000 (the New Dutch List) ANNEXES Circular on target values and intervention values for soil remediation Four annexes belong to this circular: · annex A deals with the target values, the soil remediation intervention values and the indicative levels for serious contamination; · annex B contains the measurement and analysis regulations for soil/sediment and groundwater for the substances listed in annex A; · annex C gives the data required for determining the remediation urgency and the remediation deadline for the substances in part A; · annex D provides a guideline for dealing with substances for which there are no standards. Circular on target values and intervention values for soil remediation page 1 of 51 www.esdat.net Esdat Environmental Database Management Software +61 2 9232 8080 ANNEX A: TARGET VALUES, SOIL REMEDIATION INTERVENTION VALUES AND INDICATIVE LEVELS FOR SERIOUS CONTAMINATION Introduction Soil remediation policy uses soil remediation intervention values, indicative levels for serious contamination and target values. These three types of standards are dealt with below. The point of departure in setting standards for environmental policy as a whole is the risks involved. This strategy is set forth in the document Premises for Risk Management [Omgaan met risico’s]. The risk-based approach in environmental policy (Ministry of Housing, Spatial Planning and the Environment (VROM), Lower House of Parliament, parliamentary proceedings 1988-1989, 21 137, no. 5). The intervention values and the accompanying target values for soil/sediment and groundwater are given in table 1.