AP-42, Vol. 1, Final Background Document for Pesticide Application
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Restricted Use Product Summary Report
Page 1 of 17 Restricted Use Product Summary Report (January 19, 2016) Percent Active Registration # Name Company # Company Name Active Ingredient(s) Ingredient 4‐152 BONIDE ORCHARD MOUSE BAIT 4 BONIDE PRODUCTS, INC. 2 Zinc phosphide (Zn3P2) 70‐223 RIGO EXOTHERM TERMIL 70 VALUE GARDENS SUPPLY, LLC 20 Chlorothalonil 100‐497 AATREX 4L HERBICIDE 100 SYNGENTA CROP PROTECTION, LLC 42.6 Atrazine 100‐585 AATREX NINE‐O HERBICIDE 100 SYNGENTA CROP PROTECTION, LLC 88.2 Atrazine 100‐669 CURACRON 8E INSECTICIDE‐MITICIDE 100 SYNGENTA CROP PROTECTION, LLC 73 Profenofos 100‐817 BICEP II MAGNUM HERBICIDE 100 SYNGENTA CROP PROTECTION, LLC 33; 26.1 Atrazine; S‐Metolachlor 100‐827 BICEP LITE II MAGNUM HERBICIDE 100 SYNGENTA CROP PROTECTION, LLC 28.1; 35.8 Atrazine; S‐Metolachlor 100‐886 BICEP MAGNUM 100 SYNGENTA CROP PROTECTION, LLC 33.7; 26.1 Atrazine; S‐Metolachlor 100‐898 AGRI‐MEK 0.15 EC MITICIDE/INSECTICIDE 100 SYNGENTA CROP PROTECTION, LLC 2 Abamectin 100‐903 DENIM INSECTICIDE 100 SYNGENTA CROP PROTECTION, LLC 2.15 Emamectin benzoate 100‐904 PROCLAIM INSECTICIDE 100 SYNGENTA CROP PROTECTION, LLC 5 Emamectin benzoate 100‐998 KARATE 1EC 100 SYNGENTA CROP PROTECTION, LLC 13.1 lambda‐Cyhalothrin 100‐1075 FORCE 3G INSECTICIDE 100 SYNGENTA CROP PROTECTION, LLC 3 Tefluthrin Acetochlor; Carbamothioic acid, dipropyl‐ 100‐1083 DOUBLEPLAY SELECTIVE HERBICIDE 100 SYNGENTA CROP PROTECTION, LLC 16.9; 67.8 , S‐ethyl ester 100‐1086 KARATE EC‐W INSECTICIDE 100 SYNGENTA CROP PROTECTION, LLC 13.1 lambda‐Cyhalothrin 100‐1088 SCIMITAR GC INSECTICIDE 100 SYNGENTA CROP PROTECTION, -

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Common and Chemical Names of Herbicides Approved by the WSSA
Weed Science 2010 58:511–518 Common and Chemical Names of Herbicides Approved by the Weed Science Society of America Below is the complete list of all common and chemical of herbicides as approved by the International Organization names of herbicides approved by the Weed Science Society of for Standardization (ISO). A sponsor may submit a proposal America (WSSA) and updated as of September 1, 2010. for a common name directly to the WSSA Terminology Beginning in 1996, it has been published yearly in the last Committee. issue of Weed Science with Directions for Contributors to A herbicide common name is not synonymous with Weed Science. This list is published in lieu of the selections a commercial formulation of the same herbicide, and in printed previously on the back cover of Weed Science. Only many instances, is not synonymous with the active ingredient common and chemical names included in this complete of a commercial formulation as identified on the product list should be used in WSSA publications. In the absence of label. If the herbicide is a salt or simple ester of a parent a WSSA-approved common name, the industry code number compound, the WSSA common name applies to the parent as compiled by the Chemical Abstracts Service (CAS) with compound only. CAS systematic chemical name or the systematic chemical The chemical name used in this list is that preferred by the name alone may be used. The current approved list is also Chemical Abstracts Service (CAS) according to their system of available at our web site (www.wssa.net). -

Growth Regulation and Other Secondary Effects of Herbicides Edivaldo D
Weed Science 2010 58:351–354 Growth Regulation and Other Secondary Effects of Herbicides Edivaldo D. Velini, Maria L. B. Trindade, Luis Rodrigo M. Barberis, and Stephen O. Duke* As all herbicides act on pathways or processes crucial to plants, in an inhibitory or stimulatory way, low doses of any herbicide might be used to beneficially modulate plant growth, development, or composition. Glyphosate, the most used herbicide in the world, is widely applied at low rates to ripen sugarcane. Low rates of glyphosate also can stimulate plant growth (this effect is called hormesis). When applied at recommended rates for weed control, glyphosate can inhibit rust diseases in glyphosate-resistant wheat and soybean. Fluridone blocks carotenoid biosynthesis by inhibition of phytoene desaturase and is effective in reducing the production of abscisic acid in drought-stressed plants. Among the acetolactate synthase inhibitors, sulfometuron-methyl is widely used to ripen sugarcane and imidazolinones can be used to suppress turf species growth. The application of protoporphyrinogen oxidase inhibitors can trigger plant defenses against pathogens. Glufosinate, a glutamine synthetase inhibitor, is also known to improve the control of plant diseases. Auxin agonists (i.e., dicamba and 2,4-D) are effective, low-cost plant growth regulators. Currently, auxin agonists are still used in tissue cultures to induce somatic embryogenesis and to control fruit ripening, to reduce drop of fruits, to enlarge fruit size, or to extend the harvest period in citrus orchards. At low doses, triazine herbicides stimulate growth through beneficial effects on nitrogen metabolism and through auxin-like effects. Thus, sublethal doses of several herbicides have applications other than weed control. -

Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries
Historical Perspectives on Apple Production: Fruit Tree Pest Management, Regulation and New Insecticidal Chemistries. Peter Jentsch Extension Associate Department of Entomology Cornell University's Hudson Valley Lab 3357 Rt. 9W; PO box 727 Highland, NY 12528 email: [email protected] Phone 845-691-7151 Mobile: 845-417-7465 http://www.nysaes.cornell.edu/ent/faculty/jentsch/ 2 Historical Perspectives on Fruit Production: Fruit Tree Pest Management, Regulation and New Chemistries. by Peter Jentsch I. Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 Synthetic Pesticide Development and Use II. Influences Changing the Pest Management Profile in Apple Production Chemical Residues in Early Insect Management Historical Chemical Regulation Recent Regulation Developments Changing Pest Management Food Quality Protection Act of 1996 The Science Behind The Methodology Pesticide Revisions – Requirements For New Registrations III. Resistance of Insect Pests to Insecticides Resistance Pest Management Strategies IV. Reduced Risk Chemistries: New Modes of Action and the Insecticide Treadmill Fermentation Microbial Products Bt’s, Abamectins, Spinosads Juvenile Hormone Analogs Formamidines, Juvenile Hormone Analogs And Mimics Insect Growth Regulators Azadirachtin, Thiadiazine Neonicotinyls Major Reduced Risk Materials: Carboxamides, Carboxylic Acid Esters, Granulosis Viruses, Diphenyloxazolines, Insecticidal Soaps, Benzoyl Urea Growth Regulators, Tetronic Acids, Oxadiazenes , Particle Films, Phenoxypyrazoles, Pyridazinones, Spinosads, Tetrazines , Organotins, Quinolines. 3 I Historical Use of Pesticides in Apple Production Overview of Apple Production and Pest Management Prior to 1940 The apple has a rather ominous origin. Its inception is framed in the biblical text regarding the genesis of mankind. The backdrop appears to be the turbulent setting of what many scholars believe to be present day Iraq. -

Acifluorfen Sorption, Degradation, and Mobility in a Mississippi Delta Soil
Acifluorfen Sorption, Degradation, and Mobility in a Mississippi Delta Soil L. A. Gaston* and M. A. Locke ABSTRACT repulsion effects, acifluorfen is sorbed by soil or soil Potential surface water and groundwater contaminants include her- constituents (Pusino et al., 1991; Ruggiero et al., 1992; bicides that are applied postemergence. Although applied to the plant Pusino et al., 1993; Gennari et al., 1994b; NeÁgre et al., canopy, a portion of any application reaches the soil either directly 1995; Locke et al., 1997). Although the extent of sorp- or via subsequent foliar washoff. This study examined sorption, degra- tion in soil is generally proportional to OC content dation, and mobility of the postemergence herbicide acifluorfen (5-[2- (Gennari et al., 1994b; NeÁgre et al., 1995; Locke et al., chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid) in Dundee 1997), sorption likely involves processes other than par- silty clay loam (fine-silty, mixed, thermic, Aeric Ochraqualf) taken titioning between aqueous and organic matter phases. from conventional till (CT) and no-till (NT) field plots. Homogeneous In particular, acifluorfen forms complexes with divalent surface and subsurface samples were used in the sorption and degrada- tion studies; intact soil columns (30 cm long and 10 cm diam.) were and trivalent cations (Pusino et al., 1991; Pusino et al., used in the mobility study. Batch sorption isotherms were nonlinear 1993) that may be sorbed or precipitated. Complex for- (Freundlich model) and sorption paralleled organic C (OC) content. mation and subsequent sorption may partially account All tillage by depth combinations of soil exhibited a time-dependent for increased acifluorfen sorption with decreasing soil approach to sorption equilibrium that was well described by a two- pH or increasing cation exchange capacity (Pusino et site equilibrium±kinetic model. -

40 CFR Ch. I (7–1–18 Edition) § 455.61
§ 455.61 40 CFR Ch. I (7–1–18 Edition) from: the operation of employee show- § 455.64 Effluent limitations guidelines ers and laundry facilities; the testing representing the degree of effluent of fire protection equipment; the test- reduction attainable by the applica- ing and emergency operation of safety tion of the best available tech- showers and eye washes; or storm nology economically achievable water. (BAT). (d) The provisions of this subpart do Except as provided in 40 CFR 125.30 not apply to wastewater discharges through 125.32, any existing point from the repackaging of microorga- source subject to this subpart must nisms or Group 1 Mixtures, as defined achieve effluent limitations rep- under § 455.10, or non-agricultural pes- resenting the degree of effluent reduc- ticide products. tion attainable by the application of the best available technology economi- § 455.61 Special definitions. cally achievable: There shall be no dis- Process wastewater, for this subpart, charge of process wastewater pollut- means all wastewater except for sani- ants. tary water and those wastewaters ex- § 455.65 New source performance cluded from the applicability of the standards (NSPS). rule in § 455.60. Any new source subject to this sub- § 455.62 Effluent limitations guidelines part which discharges process waste- representing the degree of effluent water pollutants must meet the fol- reduction attainable by the applica- lowing standards: There shall be no dis- tion of the best practicable pollut- charge of process wastewater pollut- ant control technology (BPT). ants. Except as provided in 40 CFR 125.30 through 125.32, any existing point § 455.66 Pretreatment standards for existing sources (PSES). -

Agricultural Biotechnology: Benefits of Transgenic Soybeans
AGRICULTURAL BIOTECHNOLOGY: BENEFITS OF TRANSGENIC SOYBEANS Leonard P. Gianessi Janet E. Carpenter April 2000 National Center for Food and Agricultural Policy 1616 P Street, NW, First Floor Washington, DC 20036 Tel: 202-328-5048 Fax: 202-328-5133 [email protected] Preparation of this report was supported financially with a grant from the Rockefeller Foundation TABLE OF CONTENTS 1. Introduction 2. U.S. Soybean Production 3. Soybean Products 4. Soybean Physiology 5. Soybeans – Agronomic Factors 6. Soybean Genetic Improvements A. Introduction B. Reproductive Process C. Artificial Cross Breeding D. Mutation Breeding E. Transgenic Plants 7. Weed Competition – Soybeans 8. Weed Control in Soybeans: 1940’s – 1950’s 9. Herbicides – An Overview 10. Herbicide Use in Soybeans: 1960’s – 1995 A. Introduction B. Historical Overview 1. The Early 1960’s 2. Soil Applied Herbicides 3. Postemergence Herbicides 4. Sulfonylurea/Imidazolinone Herbicides 5. Burndown Herbicides C. Summary of Usage: 1995 11. Transgenic Herbicide Tolerant Soybeans A. Glyphosate – An Overview B. Performance of Roundup Ready Soybeans C. Herbicide Ratings D. Adoption Impacts: 1995 – 1998 1. Herbicide Costs 2. Soybean Yields 3. Returns 4. Other Aggregate Studies 5. Herbicide Treatments 6. Herbicide Use Amounts 7. Other Impacts 12. Summary and Conclusions 13. References Appendix 1: Soybean Processing – A Description 1. Introduction Soybeans and other crops have been improved genetically for many decades through traditional crop breeding – a technique that requires that species be sexually compatible. With the development of biotechnology methods, scientists have the ability to transfer single genes from one living organism into another, regardless of species or sexual compatibility. Varieties that are developed through the transfer of genes between species that are not sexually compatible are referred to as “transgenic.” Transgenic soybean plants have been developed with a gene from a soil bacteria that allows the use of an herbicide that would normally kill soybeans. -

A Novel Role of Ethephon in Controlling the Noxious Weed Ipomoea Cairica
www.nature.com/scientificreports OPEN A novel role of ethephon in controlling the noxious weed Ipomoea cairica (Linn.) Sweet Received: 09 April 2015 1,* 1,* 1,3,* 2 1 Accepted: 22 May 2015 Zhong-Yu Sun , Tai-Jie Zhang , Jin-Quan Su , Wah Soon Chow , Jia-Qin Liu , 1 1 3 1 Published: 18 June 2015 Li-Ling Chen , Wei-Hua Li , Shao-Lin Peng & Chang-Lian Peng Several auxin herbicides, such as 2, 4-D and dicamba, have been used to eradicate an exotic invasive weed Ipomoea cairica in subtropical China, but restraining the re-explosion of this weed is still a challenge. Since ethylene is one of the major intermediate functioning products during the eradication process, we explored the possibility, mechanism and efficiency of using ethephon which can release ethylene to control Ipomoea cairica. The results of the pot experiment showed that 7.2 g /L ethephon could totally kill Ipomoea cairica including the stems and roots. The water culture experiment indicated that ethephon released an abundance of ethylene directly in leaves and caused increases in electrolyte leakage, 1-aminocyclopropane-1-carboxylic acid (ACC), abscisic acid (ABA) and H2O2 and decreases in chlorophyll content and photosynthetic activity, finally leading to the death of Ipomoea cairica. The field experiment showed that the theoretical effective concentration of ethephon for controlling Ipomoea cairica (weed control efficacy, WCE = 98%) was 4.06 g/L and the half inhibitory concentration (I50) was 0.56 g/L. More than 50% of the accompanying species were insensitive to the phytotoxicity of ethephon. Therefore, ethephon is an excellent alternative herbicide for controlling Ipomoea cairica. -

INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES
US Environmental Protection Agency Office of Pesticide Programs INDEX to PESTICIDE TYPES and FAMILIES and PART 180 TOLERANCE INFORMATION of PESTICIDE CHEMICALS in FOOD and FEED COMMODITIES Note: Pesticide tolerance information is updated in the Code of Federal Regulations on a weekly basis. EPA plans to update these indexes biannually. These indexes are current as of the date indicated in the pdf file. For the latest information on pesticide tolerances, please check the electronic Code of Federal Regulations (eCFR) at http://www.access.gpo.gov/nara/cfr/waisidx_07/40cfrv23_07.html 1 40 CFR Type Family Common name CAS Number PC code 180.163 Acaricide bridged diphenyl Dicofol (1,1-Bis(chlorophenyl)-2,2,2-trichloroethanol) 115-32-2 10501 180.198 Acaricide phosphonate Trichlorfon 52-68-6 57901 180.259 Acaricide sulfite ester Propargite 2312-35-8 97601 180.446 Acaricide tetrazine Clofentezine 74115-24-5 125501 180.448 Acaricide thiazolidine Hexythiazox 78587-05-0 128849 180.517 Acaricide phenylpyrazole Fipronil 120068-37-3 129121 180.566 Acaricide pyrazole Fenpyroximate 134098-61-6 129131 180.572 Acaricide carbazate Bifenazate 149877-41-8 586 180.593 Acaricide unclassified Etoxazole 153233-91-1 107091 180.599 Acaricide unclassified Acequinocyl 57960-19-7 6329 180.341 Acaricide, fungicide dinitrophenol Dinocap (2, 4-Dinitro-6-octylphenyl crotonate and 2,6-dinitro-4- 39300-45-3 36001 octylphenyl crotonate} 180.111 Acaricide, insecticide organophosphorus Malathion 121-75-5 57701 180.182 Acaricide, insecticide cyclodiene Endosulfan 115-29-7 79401 -

4. Chemical and Physical Information
PYRETHRINS AND PYRETHROIDS 131 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY The naturally-occurring pyrethrins, extracted from chrysanthemum flowers, are esters of chrysanthemic acid (Pyrethrin I, Cinerin I, and Jasmolin I) and esters of pyrethric acid (Pyrethrin II, Cinerin II, and Jasmolin II). In the United States, the pyrethrum extract is standardized as 45–55% w/w total pyrethrins. The typical proportion of Pyrethrins I to II is 0.2:2.8, while the ratio of pyrethrins:cinerins:jasmolins is 71:21:7 (Tomlin 1997). Information regarding the chemical identity of the pyrethrins is presented in Table 4-1. Pyrethroids are synthetic esters derived from the naturally-occurring pyrethrins. One exception to the axiom that all pyrethroids are esters of carboxylic acids is noteworthy. There is a group of oxime ethers that exhibits insecticidal activity similar in nature to the pyrethrins and pyrethroid esters (Davies 1985). Little data exist regarding these compounds, and no commercial products have been produced. Commercially available pyrethroids include allethrin, bifenthrin, bioresmethrin, cyfluthrin, cyhalothrin, cypermethrin, deltamethrin, esfenvalerate (fenvalerate), flucythrinate, flumethrin, fluvalinate, fenpropathrin, permethrin, phenothrin, resmethrin, tefluthrin, tetramethrin, and tralomethrin. Information regarding the chemical identity of pyrethroids is shown in Table 4-2. With the exception of deltamethrin, pyrethroids are a complex mixture of isomers rather than one single pure compound. For pyrethroids possessing the cyclopropane moiety, isomerism about the cyclopropane ring greatly influences the toxicity of these insecticides. The presence of two chiral centers in the ring results in two pairs of diastereomers. The diastereomers and their nonsuperimposable mirror images (enantiomers) are illustrated in Figure 4-1. -
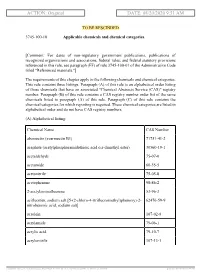
ACTION: Original DATE: 08/20/2020 9:51 AM
ACTION: Original DATE: 08/20/2020 9:51 AM TO BE RESCINDED 3745-100-10 Applicable chemicals and chemical categories. [Comment: For dates of non-regulatory government publications, publications of recognized organizations and associations, federal rules, and federal statutory provisions referenced in this rule, see paragraph (FF) of rule 3745-100-01 of the Administrative Code titled "Referenced materials."] The requirements of this chapter apply to the following chemicals and chemical categories. This rule contains three listings. Paragraph (A) of this rule is an alphabetical order listing of those chemicals that have an associated "Chemical Abstracts Service (CAS)" registry number. Paragraph (B) of this rule contains a CAS registry number order list of the same chemicals listed in paragraph (A) of this rule. Paragraph (C) of this rule contains the chemical categories for which reporting is required. These chemical categories are listed in alphabetical order and do not have CAS registry numbers. (A) Alphabetical listing: Chemical Name CAS Number abamectin (avermectin B1) 71751-41-2 acephate (acetylphosphoramidothioic acid o,s-dimethyl ester) 30560-19-1 acetaldehyde 75-07-0 acetamide 60-35-5 acetonitrile 75-05-8 acetophenone 98-86-2 2-acetylaminofluorene 53-96-3 acifluorfen, sodium salt [5-(2-chloro-4-(trifluoromethyl)phenoxy)-2- 62476-59-9 nitrobenzoic acid, sodium salt] acrolein 107-02-8 acrylamide 79-06-1 acrylic acid 79-10-7 acrylonitrile 107-13-1 [ stylesheet: rule.xsl 2.14, authoring tool: RAS XMetaL R2_0F1, (dv: 0, p: 185720, pa: