Agricultural Biotechnology: Benefits of Transgenic Soybeans
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Roundup Ready Wheat – an Overview Based on Advancements in the Risk Assessment of Genetically Engineered Crops
Roundup Ready Wheat – An Overview Based on Advancements in the Risk Assessment of Genetically Engineered Crops by Doug Gurian-Sherman, Ph.D. Center for Science in the Public Interest 1875 Connecticut Avenue, N.W., Suite 300 Washington, DC 20009-5728 Phone: (202) 332-9110 www.cspinet.org TABLE OF CONTENTS SECTION PAGE Abstract................................................................................................................................. 2 Introduction.......................................................................................................................... 3 Background on the U.S. Regulatory System for GE Crops ............................................. 3 Characterization of the Transgene and Transgenic Protein............................................ 4 Human Safety....................................................................................................................... 6 Allergenicity ...................................................................................................................... 7 Unintended Adverse Effects.............................................................................................. 9 Environmental Issues ........................................................................................................ 11 Resistance Management ................................................................................................. 12 Gene Transfer.............................................................................................................. -

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Common and Chemical Names of Herbicides Approved by the WSSA
Weed Science 2010 58:511–518 Common and Chemical Names of Herbicides Approved by the Weed Science Society of America Below is the complete list of all common and chemical of herbicides as approved by the International Organization names of herbicides approved by the Weed Science Society of for Standardization (ISO). A sponsor may submit a proposal America (WSSA) and updated as of September 1, 2010. for a common name directly to the WSSA Terminology Beginning in 1996, it has been published yearly in the last Committee. issue of Weed Science with Directions for Contributors to A herbicide common name is not synonymous with Weed Science. This list is published in lieu of the selections a commercial formulation of the same herbicide, and in printed previously on the back cover of Weed Science. Only many instances, is not synonymous with the active ingredient common and chemical names included in this complete of a commercial formulation as identified on the product list should be used in WSSA publications. In the absence of label. If the herbicide is a salt or simple ester of a parent a WSSA-approved common name, the industry code number compound, the WSSA common name applies to the parent as compiled by the Chemical Abstracts Service (CAS) with compound only. CAS systematic chemical name or the systematic chemical The chemical name used in this list is that preferred by the name alone may be used. The current approved list is also Chemical Abstracts Service (CAS) according to their system of available at our web site (www.wssa.net). -

Harness Xtra Herbicide, EPA Registration REPACKAGING LIMITATIONS
ATTENTION: This specimen label is provided for general information only. • This pesticide product may not yet be available or approved for sale or use in your area. • It is your responsibility to follow all Federal, state and local laws and regulations regarding the use of pesticides. • Before using any pesticide, be sure the intended use is approved in your state or locality. • Your state or locality may require additional precautions and instructions for use of this product that are not included here. • Monsanto does not guarantee the completeness or accuracy of this specimen label. The information found in this label may differ from the information found on the product label. You must have the EPA approved labeling with you at the time of use and must read and follow all label directions. • You should not base any use of a similar product on the precautions, instructions for use or other information you find here. • Always follow the precautions and instructions for use on the label of the pesticide you are using. 36021K6-29 3.0 PRECAUTIONARY STATEMENTS RESTRICTED USE PESTICIDE due to ground and surface water concerns. For retail sale to and use only by .1 Hazards to Humans and Domestic Animals Certified Applicators, or persons under their direct supervision and only for those 3 uses covered by the Certified Applicator’s certification. Keep out of reach of children. CAUTION! HARMFUL IF SWALLOWED. HARMFUL IF INHALED. CAUSES MODERATE EYE IRRITATION. ® MAY CAUSE ALLERGIC SKIN REACTION. Avoid contact with skin, eyes, or clothing. Avoid breathing spray mist. Prolonged or frequently repeated skin contact may cause allergic reactions in some individuals. -
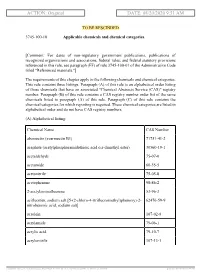
ACTION: Original DATE: 08/20/2020 9:51 AM
ACTION: Original DATE: 08/20/2020 9:51 AM TO BE RESCINDED 3745-100-10 Applicable chemicals and chemical categories. [Comment: For dates of non-regulatory government publications, publications of recognized organizations and associations, federal rules, and federal statutory provisions referenced in this rule, see paragraph (FF) of rule 3745-100-01 of the Administrative Code titled "Referenced materials."] The requirements of this chapter apply to the following chemicals and chemical categories. This rule contains three listings. Paragraph (A) of this rule is an alphabetical order listing of those chemicals that have an associated "Chemical Abstracts Service (CAS)" registry number. Paragraph (B) of this rule contains a CAS registry number order list of the same chemicals listed in paragraph (A) of this rule. Paragraph (C) of this rule contains the chemical categories for which reporting is required. These chemical categories are listed in alphabetical order and do not have CAS registry numbers. (A) Alphabetical listing: Chemical Name CAS Number abamectin (avermectin B1) 71751-41-2 acephate (acetylphosphoramidothioic acid o,s-dimethyl ester) 30560-19-1 acetaldehyde 75-07-0 acetamide 60-35-5 acetonitrile 75-05-8 acetophenone 98-86-2 2-acetylaminofluorene 53-96-3 acifluorfen, sodium salt [5-(2-chloro-4-(trifluoromethyl)phenoxy)-2- 62476-59-9 nitrobenzoic acid, sodium salt] acrolein 107-02-8 acrylamide 79-06-1 acrylic acid 79-10-7 acrylonitrile 107-13-1 [ stylesheet: rule.xsl 2.14, authoring tool: RAS XMetaL R2_0F1, (dv: 0, p: 185720, pa: -

Lack of Glyphosate Resistance Gene Transfer from Roundup Ready
Lack of Glyphosate Resistance Gene Transfer from Roundup Ready® Soybean to Bradyrhizobium japonicum under Field and Laboratory Conditions Laura Arango Isazaa, Katja Opelta, Tobias Wagnera,b, Elke Mattesa, Evi Biebera, Elwood O. Hatleyc, Greg Rothc, Juan Sanjuánd, Hans-Martin Fischere, Heinrich Sandermanna, Anton Hartmannf, and Dieter Ernsta,* a Institute of Biochemical Plant Pathology, Helmholtz Zentrum München – German Research Center for Environmental Health, D-85764 Neuherberg, Germany. Fax: +49 89 3187 3383. E-mail: [email protected] b Present address: Applied Biosystems, Frankfurter Strasse 129B, D-64293 Darmstadt, Germany c The Pennsylvania State University, Department of Crop and Soil Sciences, University Park, PA 16802, USA d Departamento de Microbiologia del Suelo y Sistemas Simbióticos, Estación Experimental del Zaidin, CSIC, Prof. Albareda 1, Granada, Spain e ETH, Institute of Microbiology, Wolfgang-Pauli-Str. 10, Zürich, Switzerland f Department Microbe-Plant Interactions, Helmholtz Zentrum München – German Research Center for Environmental Health, D-85764 Neuherberg, Germany * Author for correspondence and reprint requests Z. Naturforsch. 66 c, 595 – 604 (2011); received March 25/October 14, 2011 This paper is dedicated to the memory of the late Professor Dr. Heinrich Sandermann, former director of the Institute of Biochemical Plant Pathology, Helmholtz Zentrum München A fi eld study was conducted at the Russell E. Larson Agricultural Research Center to de- termine the effect of transgenic glyphosate-resistant soybean in combination with herbicide (Roundup) application on its endosymbiont Bradyrhizobium japonicum. DNA of bacteroids from isolated nodules was analysed for the presence of the transgenic 5-enolpyruvylshiki- mate-3-phosphate synthase (CP4-EPSPS) DNA sequence using polymerase chain reaction (PCR). -

Sustainable Intensive Agriculture: High Technology and Environmental Benefits
University of Arkansas School of Law [email protected] $ (479) 575-7646 An Agricultural Law Research Article Sustainable Intensive Agriculture: High Technology and Environmental Benefits by Drew L. Kershen Originally published in KANSAS JOURNAL OF LAW AND PUBLIC POLICY 16 KAN. J. L. & PUB. POL’Y 424 (2007) www.NationalAgLawCenter.org SUSTAINABLE INTENSIVE AGRICULTURE: HIGH TECHNOLOGY AND ENVIRONMENTAL BENEFITS Drew L. Kershen- I. PREFACE In the coming decades, agriculture faces three significant challenges. While these challenges will manifest themselves in ways unique to the cultural, socio-economic, and political conditions of different countries, developed and developing nations alike will face these challenges. Moreover, for the purposes of this article, the author assumes these challenges are truisms; consequently, there is no need to cite authority to support the author's identification and assertions. 1 Agriculture faces an agronomic challenge. Millions of people are still hungry in our world. Moreover, the world's population will continue to grow . for at least several decades. Agriculture must produce the food necessary to provide the people of the world-including those who presently have the money to feel secure about their daily bread-with an adequate supply of nutritious food'. "Agriculture must first be about food production for the survival and health of human beings, Agriculture faces an environmental challenge. It cannot produce the food needed for human beings if it exhausts or abuses Earth's soil, water, air, and biodiversity. Moreover, the general public, governments, and civil organizations from all societal sectors (academic, business, consumer, for profit and non-profit, public interest, and scientific) demand that agriculture Earl Sneed Centennial Professor of Law, University of Oklahoma, College of Law. -

World Resources Institute the Monsanto Company
World Resources Institute Sustainable Enterprise Program A program of the World Resources Institute The Monsanto Company: Quest for Sustainability (A) “Biotechnology represents a potentially sustainable For more than a decade, WRI's solution to the issue, not only of feeding people, but of providing Sustainable Enterprise Program (SEP) the economic growth that people are going to need to escape has harnessed the power of business to poverty…… [Biotechnology] poses the possibility of create profitable solutions to leapfrogging the industrial revolution and moving to a post- environment and development industrial society that is not only economically attractive, but challenges. BELL, a project of SEP, is also environmentally sustainable.i ” focused on working with managers and academics to make companies --Robert Shapiro, CEO, Monsanto Company more competitive by approaching social and environmental challenges as unmet market needs that provide Upon his promotion to CEO of chemical giant The business growth opportunities through Monsanto Company in 1995, Robert Shapiro became a vocal entrepreneurship, innovation, and champion of sustainable development and sought to redefine the organizational change. firm’s business strategy along principles of sustainability. Shapiro’s rhetoric was compelling. He captured analysts’ Permission to reprint this case is attention with the specter of mass hunger and environmental available at the BELL case store. degradation precipitated by rapid population growth and the Additional information on the Case -

Glyphosate and Cancer Risk: Frequently Asked Questions
May 2015 FACT SHEET GLYPHOSATE AND CANCER RISK: FREQUENTLY ASKED QUESTIONS WHY IS THERE CONCERN ABOUT showing higher rates of cancer in glyphosate-using GLYPHOSATE AND CANCER? The World farmers; and research showing that glyphosate damages Health Organization’s (WHO’s) cancer authorities – the DNA and chromosomes, one mechanism by which cancer International Agency for Research on Cancer (IARC) is induced.2 IARC’s full assessment is due out in 2016. – recently determined that glyphosate is “probably carcinogenic to humans” (Group 2A). Glyphosate is WHOSE ASSESSMENT IS MORE the most heavily used pesticide in the world thanks to RELIABLE: IARC OR EPA? IARC is the world’s widespread planting of Monsanto’s Roundup Ready crops, leading authority on cancer. Its glyphosate determination which are genetically engineered to survive spraying with was made by unanimous decision of 17 qualified scientists it. Use and exposure will increase still more if glyphosate- led by Dr. Aaron Blair, a distinguished epidemiologist resistant turfgrasses currently being developed for lawns, recently retired from the U.S. National Cancer Institute.3 playing fields and golf courses are introduced. IARC’s assessment is up-to-date, analyzing all the relevant available research, while EPA’s last comprehensive WHERE DO EPA AND WHO’S IARC STAND assessment of glyphosate occurred in 1993. IARC ON GLYPHOSATE’S CARCINOGENICITY? considered a broad range of evidence, including human In 1985, EPA classified glyphosate as a possible epidemiology and other peer-reviewed studies, while EPA carcinogen based on experiments showing tumors in did not assess epidemiology and relied almost entirely on glyphosate-treated rodents. -

Monsanto Improved Fatty Acid Profile MON 87705 Soybean, Petition 09-201-01P
Monsanto Improved Fatty Acid Profile MON 87705 Soybean, Petition 09-201-01p OECD Unique Identifier: MON-87705-6 Final Environmental Assessment September 2011 Agency Contact Cindy Eck USDA, APHIS, BRS 4700 River Road, Unit 147 Riverdale, MD 20737-1237 Phone: (301) 734-0667 Fax: (301) 734-8669 [email protected] The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, or marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’S TARGET Center at (202) 720–2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326–W, Whitten Building, 1400 Independence Avenue, SW, Washington, DC 20250–9410 or call (202) 720–5964 (voice and TDD). USDA is an equal opportunity provider and employer. Mention of companies or commercial products in this report does not imply recommendation or endorsement by the U.S. Department of Agriculture over others not mentioned. USDA neither guarantees nor warrants the standard of any product mentioned. Product names are mentioned solely to report factually on available data and to provide specific information. This publication reports research involving pesticides. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. MONSANTO 87705 SOYBEAN TABLE OF CONTENTS PAGE ACRONYMS ................................................................................................................................ iv 1 PURPOSE AND NEED ........................................................................................................ -

AP-42, CH 9.2.2: Pesticide Application
9.2.2PesticideApplication 9.2.2.1General1-2 Pesticidesaresubstancesormixturesusedtocontrolplantandanimallifeforthepurposesof increasingandimprovingagriculturalproduction,protectingpublichealthfrompest-bornediseaseand discomfort,reducingpropertydamagecausedbypests,andimprovingtheaestheticqualityofoutdoor orindoorsurroundings.Pesticidesareusedwidelyinagriculture,byhomeowners,byindustry,andby governmentagencies.Thelargestusageofchemicalswithpesticidalactivity,byweightof"active ingredient"(AI),isinagriculture.Agriculturalpesticidesareusedforcost-effectivecontrolofweeds, insects,mites,fungi,nematodes,andotherthreatstotheyield,quality,orsafetyoffood.Theannual U.S.usageofpesticideAIs(i.e.,insecticides,herbicides,andfungicides)isover800millionpounds. AiremissionsfrompesticideusearisebecauseofthevolatilenatureofmanyAIs,solvents, andotheradditivesusedinformulations,andofthedustynatureofsomeformulations.Mostmodern pesticidesareorganiccompounds.EmissionscanresultdirectlyduringapplicationorastheAIor solventvolatilizesovertimefromsoilandvegetation.Thisdiscussionwillfocusonemissionfactors forvolatilization.Thereareinsufficientdataavailableonparticulateemissionstopermitemission factordevelopment. 9.2.2.2ProcessDescription3-6 ApplicationMethods- Pesticideapplicationmethodsvaryaccordingtothetargetpestandtothecroporothervalue tobeprotected.Insomecases,thepesticideisapplieddirectlytothepest,andinotherstothehost plant.Instillothers,itisusedonthesoilorinanenclosedairspace.Pesticidemanufacturershave developedvariousformulationsofAIstomeetboththepestcontrolneedsandthepreferred -

Benefits and Safety of Glyphosate
TABLE OF CONTENTS 1 EXECUTIVE SUMMARY ………………………………………………………………….4 2 BENEFITS OF GLYPHOSATE …………………………………………………………...5 2.1 Benefits to agriculture in glyphosate-tolerant cropping systems …………………………..6 2.1.1 Expansion in agriculture and replacement of other herbicides ……………………. 6 2.1.2 Farm level benefits ………………………………………………………………………7 2.1.3 Impacts on conservation tillage ……………………………………………………….8 2.1.4 Value of U.S. commodity exports of glyphosate-tolerant crops …………………..10 2.2 Benefits to agriculture in non-glyphosate-tolerant crops ………………………………….11 2.2.1 Orchards and vineyards ………………………………………………………………11 2.2.2 Wheat …………………………………………………………………………………..12 2.2.3 Sugarcane ……………………………………………………………………………..12 2.2.4 Cover crops ……………………………………………………………………………13 2.3 Benefits outside of agriculture ……………………………………………………………….14 2.3.1 Highway, railroad and utility right of ways …………………………………………...14 2.3.2 Recreational settings ………………………………………………………………….15 2.3.3 Invasive and noxious weeds ………………………………………………………….16 2.3.4 Aquatic weeds …………………………………………………………………………18 2.4 Managing herbicide resistant weed biotypes ……………………………………………… 19 2.5 Potential impacts of losing access to glyphosate …………………………………………. 20 2.6 Policy considerations …………………………………………………………………………..21 3 SAFETY OF GLYPHOSATE ……………………………………………………………………...23 3.1 Glyphosate environmental fate and toxicology ……………………………………..24 3.2 Glyphosate ecotoxicology …………………………………………………………….24 3.3 Glyphosate and honey bees …………………………………………………………………..25 3.4 Glyphosate and soil biota ……………………………………………………………………..25