(Poaceae: Andropogoneae): a Presumed Extinct Grass from Andhra Pradesh, India
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Phylogeny of the Hubbardochloinae Including Tetrachaete (Poaceae: Chloridoideae: Cynodonteae)
Peterson, P.M., K. Romaschenko, and Y. Herrera Arrieta. 2020. A phylogeny of the Hubbardochloinae including Tetrachaete (Poaceae: Chloridoideae: Cynodonteae). Phytoneuron 2020-81: 1–13. Published 18 November 2020. ISSN 2153 733 A PHYLOGENY OF THE HUBBARDOCHLOINAE INCLUDING TETRACHAETE (CYNODONTEAE: CHLORIDOIDEAE: POACEAE) PAUL M. PETERSON AND KONSTANTIN ROMASCHENKO Department of Botany National Museum of Natural History Smithsonian Institution Washington, D.C. 20013-7012 [email protected]; [email protected] YOLANDA HERRERA ARRIETA Instituto Politécnico Nacional CIIDIR Unidad Durango-COFAA Durango, C.P. 34220, México [email protected] ABSTRACT The phylogeny of subtribe Hubbardochloinae is revisited, here with the inclusion of the monotypic genus Tetrachaete, based on a molecular DNA analysis using ndhA intron, rpl32-trnL, rps16 intron, rps16- trnK, and ITS markers. Tetrachaete elionuroides is aligned within the Hubbardochloinae and is sister to Dignathia. The biogeography of the Hubbardochloinae is discussed, its origin likely in Africa or temperate Asia. In a previous molecular DNA phylogeny (Peterson et al. 2016), the subtribe Hubbardochloinae Auquier [Bewsia Gooss., Dignathia Stapf, Gymnopogon P. Beauv., Hubbardochloa Auquier, Leptocarydion Hochst. ex Stapf, Leptothrium Kunth, and Lophacme Stapf] was found in a clade with moderate support (BS = 75, PP = 1.00) sister to the Farragininae P.M. Peterson et al. In the present study, Tetrachaete elionuroides Chiov. is included in a phylogenetic analysis (using ndhA intron, rpl32- trnL, rps16 intron, rps16-trnK, and ITS DNA markers) in order to test its relationships within the Cynodonteae with heavy sampling of species in the supersubtribe Gouiniodinae P.M. Peterson & Romasch. Chiovenda (1903) described Tetrachaete Chiov. with a with single species, T. -

Poaceae Phytoliths from the Niassa Rift, Mozambique
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/222149229 Poaceae phytoliths from the Niassa Rift, Mozambique Article in Journal of Archaeological Science · August 2010 DOI: 10.1016/j.jas.2010.03.001 CITATIONS READS 44 409 9 authors, including: Fernando Astudillo Mary Barkworth Universidad San Francisco de Quito (USFQ) Utah State University 4 PUBLICATIONS 45 CITATIONS 81 PUBLICATIONS 902 CITATIONS SEE PROFILE SEE PROFILE Tim Aaron Bennett Chris Esselmont 8 PUBLICATIONS 242 CITATIONS The University of Calgary 6 PUBLICATIONS 161 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Stipeae (no longer a major focus) View project Grasses in North America View project All content following this page was uploaded by Rahab N Kinyanjui on 19 March 2018. The user has requested enhancement of the downloaded file. Journal of Archaeological Science 37 (2010) 1953e1967 Contents lists available at ScienceDirect Journal of Archaeological Science journal homepage: http://www.elsevier.com/locate/jas Poaceae phytoliths from the Niassa Rift, Mozambique Julio Mercader a,*, Fernando Astudillo a, Mary Barkworth b, Tim Bennett a, Chris Esselmont c, Rahab Kinyanjui d, Dyan Laskin Grossman a, Steven Simpson a, Dale Walde a a Department of Archaeology, University of Calgary, 2500 University Drive N.W., Calgary, Alberta T2N 1N4, Canada b Intermountain Herbarium, Utah State University, 5305 Old Main Hill, Logan, UH 84322-5305, USA c Environics Research Group, 999 8th Street S.W., Calgary, Alberta T2R 1J5, Canada d National Museum of Kenya, Department of Earth Sciences, Palynology and Paleobotany Section, P.O. -

Phytosociological Surveys and Monitoring of the Bromatological
European Journal of Nutrition & Food Safety 13(3): 54-61, 2021; Article no.EJNFS.68728 ISSN: 2347-5641 Phytosociological Surveys and Monitoring of the Bromatological Parameters According to the Age of Regrowth of Savannah Pastures Perfectly Reconstituted in the Central Zone of Côte d'Ivoire Gouagoua Séverin Kouadja1, Adam Camille Kouamé1*, Kouakou Eugène Kouadio1, Brou Jean Kouao1 and N’Gouan Cyrille Kouassi1 1Program of Animal Production, National Centre for Agricultural Research (CNRA), Bouaké Regional Office, P.O. box 633 Bouaké 01, Bouaké, Côte d’Ivoire. Authors’ contributions This work was carried out in collaboration among all authors. Author GSK is the principal investigator of this research. Author GSK was responsible for the collection of samples and contributed to the chemical analyses. Author Adam Camille KOUAMÉ contributed to the analyses and writing of the Manuscript. Author NCK participated in the design of the study. Author BJK was responsible for the formulation of the research question and reviewed the manuscript. Author KE reviewed the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/EJNFS/2021/v13i330390 Editor(s): (1) Dr. Kristina Mastanjevic, University of Osijek, Croatia. Reviewers: (1) Alsaied Alnaimy Habeeb, Nuclear Research Center, Egypt. (2) basem soudy, Cairo University, Egypt. Complete Peer review History: http://www.sdiarticle4.com/review-history/68728 Received 25 March 2021 Original Research Article Accepted 31 May 2021 Published 08 June 2021 ABSTRACT Although pastures in central (Affouvansou) Côte d'Ivoire are abundant, signs of undernutrition are observed in the animals towards the end of wintering, possibly due to insufficient quality pastures, poor grass quality, or poor herd distribution. -
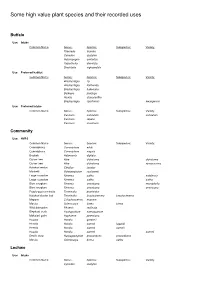
Some High Value Plant Species and Their Recorded Uses
Some high value plant species and their recorded uses Buffalo Use: Intake Common Name Genus: Species: Subspecies: Variety: Themeda triandra Cynodon dactylon Heteropogon contortus Hyperthelia dissoluta Brachiaria nigropedata Use: Preferred habitat Common Name Genus: Species: Subspecies: Variety: Brachystegia sp. Brachystegia floribunda Brachystegia bakeriana Baikiaea plurijuga Acacia ataxacantha Brachystegia spiciformis kwangensis Use: Preferred intake Common Name Genus: Species: Subspecies: Variety: Panicum coloratum coloratum Panicum repens Panicum maximum Community Use: HVPS Common Name Genus: Species: Subspecies: Variety: Commiphora Commiphora wildii Commiphora Commiphora virgata Baobab Adansonia digitata Quiver tree Aloe dichotoma dichotoma Quiver tree Aloe dichotoma ramosissima Kalahari melon Citrullus lanatus Manketti Schinziophyton rautanenii Large sourplum Ximenia caffra natalensis Large sourplum Ximenia caffra caffra Blue sourplum Ximenia americana microphylla Blue sourplum Ximenia americana americana Purple-pod terminalia Terminalia prunioides Kalahari cluster leaf Terminalia brachystemma brachystemma Mopane Colophospermu mopane Marula Sclerocarya birrea birrea Wild date palm Phoenix reclinata Elephant trunk Pachypodium namaquanum Makalani palm Hyphaene petersiana Hoodia Hoodia gordonii Hoodia Hoodia currorii lugardii Hoodia Hoodia currorii currorii Hoodia Hoodia currorii currorii Devil's claw Harpagophytum procumbens procumbens Marula Sclerocarya birrea caffra Lechwe Use: Intake Common Name Genus: Species: Subspecies: Variety: -

22. Tribe ERAGROSTIDEAE Ihl/L^Ä Huameicaozu Chen Shouliang (W-"^ G,), Wu Zhenlan (ß^E^^)
POACEAE 457 at base, 5-35 cm tall, pubescent. Basal leaf sheaths tough, whit- Enneapogon schimperianus (A. Richard) Renvoize; Pap- ish, enclosing cleistogamous spikelets, finally becoming fi- pophorum aucheri Jaubert & Spach; P. persicum (Boissier) brous; leaf blades usually involute, filiform, 2-12 cm, 1-3 mm Steudel; P. schimperianum Hochstetter ex A. Richard; P. tur- wide, densely pubescent or the abaxial surface with longer comanicum Trautvetter. white soft hairs, finely acuminate. Panicle gray, dense, spike- Perennial. Culms compactly tufted, wiry, erect or genicu- hke, linear to ovate, 1.5-5 x 0.6-1 cm. Spikelets with 3 fiorets, late, 15^5 cm tall, pubescent especially below nodes. Basal 5.5-7 mm; glumes pubescent, 3-9-veined, lower glume 3-3.5 mm, upper glume 4-5 mm; lowest lemma 1.5-2 mm, densely leaf sheaths tough, lacking cleistogamous spikelets, not becom- villous; awns 2-A mm, subequal, ciliate in lower 2/3 of their ing fibrous; leaf blades usually involute, rarely fiat, often di- length; third lemma 0.5-3 mm, reduced to a small tuft of awns. verging at a wide angle from the culm, 3-17 cm, "i-^ mm wide, Anthers 0.3-0.6 mm. PL and &. Aug-Nov. 2« = 36. pubescent, acuminate. Panicle olive-gray or tinged purplish, contracted to spikelike, narrowly oblong, 4•18 x 1-2 cm. Dry hill slopes; 1000-1900 m. Anhui, Hebei, Liaoning, Nei Mon- Spikelets with 3 or 4 florets, 8-14 mm; glumes puberulous, (5-) gol, Ningxia, Qinghai, Shanxi, Xinjiang, Yunnan [India, Kazakhstan, 7-9-veined, lower glume 5-10 mm, upper glume 7-11 mm; Kyrgyzstan, Mongolia, Pakistan, E Russia; Africa, America, SW Asia]. -

Plant Species and Functional Diversity Along Altitudinal Gradients, Southwest Ethiopian Highlands
Plant Species and Functional Diversity along Altitudinal Gradients, Southwest Ethiopian Highlands Dissertation Zur Erlangung des akademischen Grades Dr. rer. nat. Vorgelegt der Fakultät für Biologie, Chemie und Geowissenschaften der Universität Bayreuth von Herrn Desalegn Wana Dalacho geb. am 08. 08. 1973, Äthiopien Bayreuth, den 27. October 2009 Die vorliegende Arbeit wurde in dem Zeitraum von April 2006 bis October 2009 an der Universität Bayreuth unter der Leitung von Professor Dr. Carl Beierkuhnlein erstellt. Vollständiger Abdruck der von der Fakultät für Biologie, Chemie und Geowissenschaften der Universität Bayreuth zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigten Dissertation. Prüfungsausschuss 1. Prof. Dr. Carl Beierkuhnlein (1. Gutachter) 2. Prof. Dr. Sigrid Liede-Schumann (2. Gutachter) 3. PD. Dr. Gregor Aas (Vorsitz) 4. Prof. Dr. Ludwig Zöller 5. Prof. Dr. Björn Reineking Datum der Einreichung der Dissertation: 27. 10. 2009 Datum des wissenschaftlichen Kolloquiums: 21. 12. 2009 Contents Summary 1 Zusammenfassung 3 Introduction 5 Drivers of Diversity Patterns 5 Deconstruction of Diversity Patterns 9 Threats of Biodiversity Loss in the Ttropics 10 Objectives, Research Questions and Hypotheses 12 Synopsis 15 Thesis Outline 15 Synthesis and Conclusions 17 References 21 Acknowledgments 27 List of Manuscripts and Specification of Own Contribution 30 Manuscript 1 Plant Species and Growth Form Richness along Altitudinal Gradients in the Southwest Ethiopian Highlands 32 Manuscript 2 The Relative Abundance of Plant Functional Types along Environmental Gradients in the Southwest Ethiopian highlands 54 Manuscript 3 Land Use/Land Cover Change in the Southwestern Ethiopian Highlands 84 Manuscript 4 Climate Warming and Tropical Plant Species – Consequences of a Potential Upslope Shift of Isotherms in Southern Ethiopia 102 List of Publications 135 Declaration/Erklärung 136 Summary Summary Understanding how biodiversity is organized across space and time has long been a central focus of ecologists and biogeographers. -

By H.D.V. PRENDERGAST a Thesis Submitted for the Degree of Doctor of Philosophy of the Australian National University. January 1
STRUCTURAL, BIOCHEMICAL AND GEOGRAPHICAL RELATIONSHIPS IN AUSTRALIAN c4 GRASSES (POACEAE) • by H.D.V. PRENDERGAST A thesis submitted for the degree of Doctor of Philosophy of the Australian National University. January 1987. Canberra, Australia. i STATEMENT This thesis describes my own work which included collaboration with Dr N .. E. Stone (Taxonomy Unit, R .. S .. B.S .. ), whose expertise in enzyme assays enabled me to obtain comparative information on enzyme activities reported in Chapters 3, 5 and 7; and with Mr M.. Lazarides (Australian National Herbarium, c .. s .. r .. R .. O .. ), whose as yet unpublished taxonomic views on Eragrostis form the basis of some of the discussion in Chapter 3. ii This thesis describes the results of research work carried out in the Taxonomy Unit, Research School of Biological Sciences, The Australian National University during the tenure of an A.N.U. Postgraduate Scholarship. iii ACKNOWLEDGEMENTS My time in the Taxonomy Unit has been a happy one: I could not have asked for better supervision for my project or for a more congenial atmosphere in which to work. To Dr. Paul Hattersley, for his help, advice, encouragement and friendship, I owe a lot more than can be said in just a few words: but, Paul, thanks very much! To Mr. Les Watson I owe as much for his own support and guidance, and for many discussions on things often psittacaceous as well as graminaceous! Dr. Nancy Stone was a kind teacher in many days of enzyme assays and Chris Frylink a great help and friend both in and out of the lab •• Further thanks go to Mike Lazarides (Australian National Herbarium, c.s.I.R.O.) for identifying many grass specimens and for unpublished data on infrageneric groups in Eragrostis; Dr. -

Journal Vol. 30 Final 2076.7.1.Indd
102-120 J. Nat. Hist. Mus. Vol. 30, 2016-18 Flora of community managed forests of Palpa district, western Nepal Pratiksha Shrestha1, Ram Prasad Chaudhary2, Krishna Kumar Shrestha1, Dharma Raj Dangol3 1Central Department of Botany,Tribhuvan University, Kathmandu, Nepal 2Research Center for Applied Science and Technology (RECAST), Kathmandu, Nepal 3Natural History Museum, Tribhuvan University, Swayambhu, Kathmandu, Nepal ABSTRACT Floristic diversity is studied based on gender in two different management committee community forests (Barangdi-Kohal jointly managed community forest and Bansa-Gopal women managed community forest) of Palpa district, west Nepal. Square plot of 10m×10m size quadrat were laid for covering all forest areas and maintained minimum 40m distance between two quadrats. Altogether 68 plots (34 in each forest) were sampled. Both community forests had nearly same altitudinal range, aspect and slope but differed in different environmental variables and members of management committees. All the species present in quadrate and as well as outside the quadrate were recorded for analysis. There were 213 species of flowering plant belonging to 67 families and 182 genera. Barangdi-Kohal JM community forest had high species richness i.e. 176 species belonging to 64 families and 150 genera as compared to Bansa-Gopal WM community forest with 143 species belonging to 56 families and 129 genera. According to different life forms and family and genus wise jointly managed forest have high species richness than in women managed forest. Both community forests are banned for fodder, fuel wood and timber collection without permission of management comities. There is restriction of grazing in JM forest, whereas no restriction of grazing in WM forest. -

Catalogue of the Type Specimens in the National Herbarium of Cultivated Plants
e-book ISBN: 978-81-952644-1-4 Catalogue of the Type Specimens in the National Herbarium of Cultivated Plants Anjula Pandey RK Pamarthi K Pradheep Rita Gupta SP Ahlawat Division of Plant Exploration and Germplasm Collection ICAR-National Bureau of Plant Genetic Resources Pusa Campus, New Delhi - 110 012, India © 2021 ICAR-National Bureau of Plant Genetic Resources, New Delhi 110012, India This document is an outcome of taxonomic studies undertaken and new taxa described by the scientists of ICAR-National Bureau of Plant Genetic Resources (ICAR-NBPGR), New Delhi. All technical descriptions and contents on ‘type’ discussed in this publication are provided with minor modifications in the original description. Images of ‘type’ specimen have been captured and documented by the NHCP. Citation: Pandey Anjula, RK Pamarthi, K Pradheep, Rita Gupta and SP Ahlawat (2021) Catalogue of the Type Specimens in the National Herbarium of Cultivated Plants. ICAR-National Bureau of Plant Genetic Resources, New Delhi, India, 67p + i-iii Technical support: Shashi Kant Sharma Cover page photo identity: Curcuma amada var. glabra (‘type’ specimen from Kerala) Published by: The Director ICAR-National Bureau of Plant Genetic Resources New Delhi 110012, India Contact Dr. Kuldeep Singh Director ICAR-National Bureau of Plant Genetic Resources, Pusa, New Delhi 110012, India E-mail: [email protected] e-book ISBN: 978-81-952644-1-4 Catalogue of the Type Specimens in the National Herbarium of Cultivated Plants Anjula Pandey RK Pamarthi K Pradheep Rita Gupta SP Ahlawat Division of Plant Exploration and Germplasm Collection ICAR-National Bureau of Plant Genetic Resources Pusa Campus, New Delhi - 110 012, India About the book………. -

TAXONOMIC STUDIES and GENERIC DELIMITATION in the GRASS SUBTRIBE Sorghinae
TAXONOMIC STUDIES AND GENERIC DELIMITATION IN THE GRASS SUBTRIBE Sorghinae. Moffat Pinkie Setshogo A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy University of Edinburgh March 1997 Dedicated to the memory of my father, Tonkana, and to my mother, Kerileng. Acknowledgements. This work was carried out under the supervision of Dr. P.M. Smith. I wish to express my sincere gratitude to him for the advice and assistance throughout the progress of the study. I also want to thank Dr. C.E. Jeffree who has been very supportive and proof read a substantial portion of the thesis. I am indebted to the University of Botswana for the financial support and for offering me a study leave to enable me to carry out this study. The work was carried out at the Department of Botany, University of Edinburgh, as well as at the Royal Botanic Garden, Edinburgh. I would like to extend my thanks to the authorities of both institutions, and their staff, who offered help in many ways. My collection of living material was cared for by Messrs Billy Adams and Bob Astles. I wish to thank them for their help. My thanks also go to members of the photographic unit of ICMB, particularly John Anthony, Dave Haswell and Frank Johnston, for their help. Mr. John Findlay (Botany Department) gave me guidance with my SEM work, for which I am grateful. I am indebted to the Directors of various herbaria who loaned me specimens. Helen Hoy and Marisa Main were in charge of the Edinburgh side of these loans. -

Global Relationships Between Plant Functional Traits and Environment in Grasslands
GLOBAL RELATIONSHIPS BETWEEN PLANT FUNCTIONAL TRAITS AND ENVIRONMENT IN GRASSLANDS EMMA JARDINE A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy The University of Sheffield Department of Animal and Plant Sciences Submission Date July 2017 ACKNOWLEDGMENTS First of all I am enormously thankful to Colin Osborne and Gavin Thomas for giving me the opportunity to undertake the research presented in this thesis. I really appreciate all their invaluable support, guidance and advice. They have helped me to grow in knowledge, skills and confidence and for this I am extremely grateful. I would like to thank the students and post docs in both the Osborne and Christin lab groups for their help, presentations and cake baking. In particular Marjorie Lundgren for teaching me to use the Licor, for insightful discussions and general support. Also Kimberly Simpson for all her firey contributions and Ruth Wade for her moral support and employment. Thanks goes to Dave Simpson, Maria Varontsova and Martin Xanthos for allowing me to work in the herbarium at the Royal Botanic Gardens Kew, for letting me destructively harvest from the specimens and taking me on a worldwide tour of grasses. I would also like to thank Caroline Lehman for her map, her useful comments and advice and also Elisabeth Forrestel and Gareth Hempson for their contributions. I would like to thank Brad Ripley for all of his help and time whilst I was in South Africa. Karmi Du Plessis and her family and Lavinia Perumal for their South African friendliness, warmth and generosity and also Sean Devonport for sharing all the much needed teas and dub. -

Grasses and Grasslands
Grasses and Grasslands – Sensitization on the • Characteristics • Identification • Ecology • Utilisation Of Grasses by MANOJ CHANDRAN IFS Chief Conservator of Forests Govt. of Uttarakhand Grasses and Grasslands • Grass – A member of Family Poaceae • Cyperaceae- Sedges • Juncaceae - Rushes • Grassland – A vegetation community predominated by herbs and other grass or grass like plants including undergrowth trees. Temperate and Tropical Grasslands • Prairies • Pampas • Steppes • Veldt • Savanna Major Grassland types (old classification) • Dabadghao & Shankarnarayan (1973) – Sehima – Dichanthium type – Dichanthium - Cenchrus – Lasiurus type – Phragmites – Saccharum – Imperata type – Themeda – Arundinella type – Cymbopogon – Pennisetum type – Danthonia – Poa type Revised Grassland Types of India (2015) 1. Coastal Grasslands 2. Riverine Alluvial Grasslands 3. Montane Grasslands 4. Sub-Himalayan Tall Grasslands of Terai region 5. Tropical Savannas 6. Wet Grasslands Grassland types of India 1- Coastal grasslands – Sea beaches (mainland and islands) – Salt marshes – Mangrove grasslands Sea beach grasslands, Spinifex littoreus Salt marsh grasslands, Rann of Kutch 2. Riverine Alluvial grasslands Corbett NP, Katerniaghat WLS 3. Montane grasslands – Himalayan subtropical grasslands – Himalayan temperate grasslands – Alpine meadows – Trans-himalayan Steppes – Grasslands of North East Hills – Grasslands of Central Highlands – Western Ghats • Plateaus of North WG, Shola grasslands and South WG – Eastern ghats – Montane Bamboo brakes Alpine meadows, Kedarnath Alpine meadows Mamla/Ficchi – Danthonia cachemyriana Cordyceps sinensis – Yar-tsa Gam-bu Annual Migration Montane grasslands of NE Hills, Dzukou valley Montane bamboo brakes of Dzukou valley, Nagaland Shola Grasslands, South Western Ghats Pine forest grasslands, Uttarakhand Central Highlands, Pachmarhi, MP Trans-Himalayan Steppes Grazing in trans-Himalayan steppe Tibetan steppe, Mansarovar Tibetan steppe 4. Sub-himalayan Tall Grasslands of Terai Region 5.