Poaceae Phytoliths from the Niassa Rift, Mozambique
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Vascular Plant Survey of Vwaza Marsh Wildlife Reserve, Malawi
YIKA-VWAZA TRUST RESEARCH STUDY REPORT N (2017/18) Vascular Plant Survey of Vwaza Marsh Wildlife Reserve, Malawi By Sopani Sichinga ([email protected]) September , 2019 ABSTRACT In 2018 – 19, a survey on vascular plants was conducted in Vwaza Marsh Wildlife Reserve. The reserve is located in the north-western Malawi, covering an area of about 986 km2. Based on this survey, a total of 461 species from 76 families were recorded (i.e. 454 Angiosperms and 7 Pteridophyta). Of the total species recorded, 19 are exotics (of which 4 are reported to be invasive) while 1 species is considered threatened. The most dominant families were Fabaceae (80 species representing 17. 4%), Poaceae (53 species representing 11.5%), Rubiaceae (27 species representing 5.9 %), and Euphorbiaceae (24 species representing 5.2%). The annotated checklist includes scientific names, habit, habitat types and IUCN Red List status and is presented in section 5. i ACKNOLEDGEMENTS First and foremost, let me thank the Nyika–Vwaza Trust (UK) for funding this work. Without their financial support, this work would have not been materialized. The Department of National Parks and Wildlife (DNPW) Malawi through its Regional Office (N) is also thanked for the logistical support and accommodation throughout the entire study. Special thanks are due to my supervisor - Mr. George Zwide Nxumayo for his invaluable guidance. Mr. Thom McShane should also be thanked in a special way for sharing me some information, and sending me some documents about Vwaza which have contributed a lot to the success of this work. I extend my sincere thanks to the Vwaza Research Unit team for their assistance, especially during the field work. -

A Phylogeny of the Hubbardochloinae Including Tetrachaete (Poaceae: Chloridoideae: Cynodonteae)
Peterson, P.M., K. Romaschenko, and Y. Herrera Arrieta. 2020. A phylogeny of the Hubbardochloinae including Tetrachaete (Poaceae: Chloridoideae: Cynodonteae). Phytoneuron 2020-81: 1–13. Published 18 November 2020. ISSN 2153 733 A PHYLOGENY OF THE HUBBARDOCHLOINAE INCLUDING TETRACHAETE (CYNODONTEAE: CHLORIDOIDEAE: POACEAE) PAUL M. PETERSON AND KONSTANTIN ROMASCHENKO Department of Botany National Museum of Natural History Smithsonian Institution Washington, D.C. 20013-7012 [email protected]; [email protected] YOLANDA HERRERA ARRIETA Instituto Politécnico Nacional CIIDIR Unidad Durango-COFAA Durango, C.P. 34220, México [email protected] ABSTRACT The phylogeny of subtribe Hubbardochloinae is revisited, here with the inclusion of the monotypic genus Tetrachaete, based on a molecular DNA analysis using ndhA intron, rpl32-trnL, rps16 intron, rps16- trnK, and ITS markers. Tetrachaete elionuroides is aligned within the Hubbardochloinae and is sister to Dignathia. The biogeography of the Hubbardochloinae is discussed, its origin likely in Africa or temperate Asia. In a previous molecular DNA phylogeny (Peterson et al. 2016), the subtribe Hubbardochloinae Auquier [Bewsia Gooss., Dignathia Stapf, Gymnopogon P. Beauv., Hubbardochloa Auquier, Leptocarydion Hochst. ex Stapf, Leptothrium Kunth, and Lophacme Stapf] was found in a clade with moderate support (BS = 75, PP = 1.00) sister to the Farragininae P.M. Peterson et al. In the present study, Tetrachaete elionuroides Chiov. is included in a phylogenetic analysis (using ndhA intron, rpl32- trnL, rps16 intron, rps16-trnK, and ITS DNA markers) in order to test its relationships within the Cynodonteae with heavy sampling of species in the supersubtribe Gouiniodinae P.M. Peterson & Romasch. Chiovenda (1903) described Tetrachaete Chiov. with a with single species, T. -

NON-REGULATED PESTS (Non-Actionable)
Import Health Standard Commodity Sub-class: Fresh Fruit/Vegetables Grape, Vitis vinifera from Australia ISSUED Issued pursuant to Section 22 of the Biosecurity Act 1993 Date Issued: 20 December 2000 1 NEW ZEALAND NATIONAL PLANT PROTECTION ORGANISATION The official contact point in New Zealand for overseas NPPOs is the Ministry for Primary Industries (MPI). All communication pertaining to this import health standard should be addressed to: Manager, Import and Export Plants Ministry for Primary Industries PO Box 2526 Wellington NEW ZEALAND Fax: 64-4-894 0662 E-mail: [email protected] http://www.mpi.govt.nz 2 GENERAL CONDITIONS FOR ALL PLANT PRODUCTS All plants and plant products are PROHIBITED entry into New Zealand, unless an import health standard has been issued in accordance with Section 22 of the Biosecurity Act 1993. Should prohibited plants or plant products be intercepted by MPI, the importer will be offered the option of reshipment or destruction of the consignment. The national plant protection organisation of the exporting country is requested to inform MPI of any change in its address. The national plant protection organisation of the exporting country is required to inform MPI of any newly recorded organisms which may infest/infect any commodity approved for export to New Zealand. Pursuant to the Hazardous Substances and New Organisms Act 1996, proposals for the deliberate introduction of new organisms (including genetically modified organisms) as defined by the Act should be referred to: IHS Fresh Fruit/Vegetables. Grape, Vitis vinifera from Australia. (Biosecurity Act 1993) ISSUED: 20 December 2000 Page 1 of 16 Environmental Protection Authority Private Bag 63002 Wellington 6140 NEW ZEALAND Or [email protected],nz Note: In order to meet the Environmental Protection Authority requirements the scientific name (i.e. -

Phytosociological Surveys and Monitoring of the Bromatological
European Journal of Nutrition & Food Safety 13(3): 54-61, 2021; Article no.EJNFS.68728 ISSN: 2347-5641 Phytosociological Surveys and Monitoring of the Bromatological Parameters According to the Age of Regrowth of Savannah Pastures Perfectly Reconstituted in the Central Zone of Côte d'Ivoire Gouagoua Séverin Kouadja1, Adam Camille Kouamé1*, Kouakou Eugène Kouadio1, Brou Jean Kouao1 and N’Gouan Cyrille Kouassi1 1Program of Animal Production, National Centre for Agricultural Research (CNRA), Bouaké Regional Office, P.O. box 633 Bouaké 01, Bouaké, Côte d’Ivoire. Authors’ contributions This work was carried out in collaboration among all authors. Author GSK is the principal investigator of this research. Author GSK was responsible for the collection of samples and contributed to the chemical analyses. Author Adam Camille KOUAMÉ contributed to the analyses and writing of the Manuscript. Author NCK participated in the design of the study. Author BJK was responsible for the formulation of the research question and reviewed the manuscript. Author KE reviewed the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/EJNFS/2021/v13i330390 Editor(s): (1) Dr. Kristina Mastanjevic, University of Osijek, Croatia. Reviewers: (1) Alsaied Alnaimy Habeeb, Nuclear Research Center, Egypt. (2) basem soudy, Cairo University, Egypt. Complete Peer review History: http://www.sdiarticle4.com/review-history/68728 Received 25 March 2021 Original Research Article Accepted 31 May 2021 Published 08 June 2021 ABSTRACT Although pastures in central (Affouvansou) Côte d'Ivoire are abundant, signs of undernutrition are observed in the animals towards the end of wintering, possibly due to insufficient quality pastures, poor grass quality, or poor herd distribution. -

Grass Genera in Townsville
Grass Genera in Townsville Nanette B. Hooker Photographs by Chris Gardiner SCHOOL OF MARINE and TROPICAL BIOLOGY JAMES COOK UNIVERSITY TOWNSVILLE QUEENSLAND James Cook University 2012 GRASSES OF THE TOWNSVILLE AREA Welcome to the grasses of the Townsville area. The genera covered in this treatment are those found in the lowland areas around Townsville as far north as Bluewater, south to Alligator Creek and west to the base of Hervey’s Range. Most of these genera will also be found in neighbouring areas although some genera not included may occur in specific habitats. The aim of this book is to provide a description of the grass genera as well as a list of species. The grasses belong to a very widespread and large family called the Poaceae. The original family name Gramineae is used in some publications, in Australia the preferred family name is Poaceae. It is one of the largest flowering plant families of the world, comprising more than 700 genera, and more than 10,000 species. In Australia there are over 1300 species including non-native grasses. In the Townsville area there are more than 220 grass species. The grasses have highly modified flowers arranged in a variety of ways. Because they are highly modified and specialized, there are also many new terms used to describe the various features. Hence there is a lot of terminology that chiefly applies to grasses, but some terms are used also in the sedge family. The basic unit of the grass inflorescence (The flowering part) is the spikelet. The spikelet consists of 1-2 basal glumes (bracts at the base) that subtend 1-many florets or flowers. -

24. Tribe PANICEAE 黍族 Shu Zu Chen Shouliang (陈守良); Sylvia M
POACEAE 499 hairs, midvein scabrous, apex obtuse, clearly demarcated from mm wide, glabrous, margins spiny-scabrous or loosely ciliate awn; awn 1–1.5 cm; lemma 0.5–1 mm. Anthers ca. 0.3 mm. near base; ligule ca. 0.5 mm. Inflorescence up to 20 cm; spike- Caryopsis terete, narrowly ellipsoid, 1–1.8 mm. lets usually densely arranged, ascending or horizontally spread- ing; rachis scabrous. Spikelets 1.5–2.5 mm (excluding awns); Stream banks, roadsides, other weedy places, on sandy soil. Guangdong, Hainan, Shandong, Taiwan, Yunnan [Bhutan, Cambodia, basal callus 0.1–0.2 mm, obtuse; glumes narrowly lanceolate, India, Indonesia, Laos, Malaysia, Myanmar, Nepal, Philippines, Sri back scaberulous-hirtellous in rather indistinct close rows (most Lanka, Thailand, Vietnam; Africa (probably introduced), Australia obvious toward lemma base), midvein pectinate-ciliolate, apex (Queensland)]. abruptly acute, clearly demarcated from awn; awn 0.5–1.5 cm. Anthers ca. 0.3 mm. Caryopsis terete, narrowly ellipsoid, ca. 3. Perotis hordeiformis Nees in Hooker & Arnott, Bot. Beech- 1.5 mm. Fl. and fr. summer and autumn. 2n = 40. ey Voy. 248. 1838. Sandy places, along seashores. Guangdong, Hebei, Jiangsu, 麦穗茅根 mai sui mao gen Yunnan [India, Indonesia, Malaysia, Nepal, Myanmar, Pakistan, Sri Lanka, Thailand]. Perotis chinensis Gandoger. This species is very close to Perotis indica and is sometimes in- Annual or short-lived perennial. Culms loosely tufted, cluded within it. No single character by itself is reliable for separating erect or decumbent at base, 25–40 cm tall. Leaf sheaths gla- the two, but the combination of characters given in the key will usually brous; leaf blades lanceolate to narrowly ovate, 2–4 cm, 4–7 suffice. -
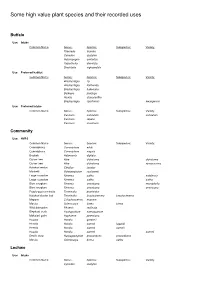
Some High Value Plant Species and Their Recorded Uses
Some high value plant species and their recorded uses Buffalo Use: Intake Common Name Genus: Species: Subspecies: Variety: Themeda triandra Cynodon dactylon Heteropogon contortus Hyperthelia dissoluta Brachiaria nigropedata Use: Preferred habitat Common Name Genus: Species: Subspecies: Variety: Brachystegia sp. Brachystegia floribunda Brachystegia bakeriana Baikiaea plurijuga Acacia ataxacantha Brachystegia spiciformis kwangensis Use: Preferred intake Common Name Genus: Species: Subspecies: Variety: Panicum coloratum coloratum Panicum repens Panicum maximum Community Use: HVPS Common Name Genus: Species: Subspecies: Variety: Commiphora Commiphora wildii Commiphora Commiphora virgata Baobab Adansonia digitata Quiver tree Aloe dichotoma dichotoma Quiver tree Aloe dichotoma ramosissima Kalahari melon Citrullus lanatus Manketti Schinziophyton rautanenii Large sourplum Ximenia caffra natalensis Large sourplum Ximenia caffra caffra Blue sourplum Ximenia americana microphylla Blue sourplum Ximenia americana americana Purple-pod terminalia Terminalia prunioides Kalahari cluster leaf Terminalia brachystemma brachystemma Mopane Colophospermu mopane Marula Sclerocarya birrea birrea Wild date palm Phoenix reclinata Elephant trunk Pachypodium namaquanum Makalani palm Hyphaene petersiana Hoodia Hoodia gordonii Hoodia Hoodia currorii lugardii Hoodia Hoodia currorii currorii Hoodia Hoodia currorii currorii Devil's claw Harpagophytum procumbens procumbens Marula Sclerocarya birrea caffra Lechwe Use: Intake Common Name Genus: Species: Subspecies: Variety: -

503 Flora V7 2.Doc 3
Browse LNG Precinct ©WOODSIDE Browse Liquefied Natural Gas Precinct Strategic Assessment Report (Draft for Public Review) December 2010 Appendix C-18 A Vegetation and Flora Survey of James Price Point: Wet Season 2009 A Vegetation and Flora Survey of James Price Point: Wet Season 2009 Prepared for Department of State Development December 2009 A Vegetation and Flora Survey of James Price Point: Wet Season 2009 © Biota Environmental Sciences Pty Ltd 2009 ABN 49 092 687 119 Level 1, 228 Carr Place Leederville Western Australia 6007 Ph: (08) 9328 1900 Fax: (08) 9328 6138 Project No.: 503 Prepared by: P. Chukowry, M. Maier Checked by: G. Humphreys Approved for Issue: M. Maier This document has been prepared to the requirements of the client identified on the cover page and no representation is made to any third party. It may be cited for the purposes of scientific research or other fair use, but it may not be reproduced or distributed to any third party by any physical or electronic means without the express permission of the client for whom it was prepared or Biota Environmental Sciences Pty Ltd. This report has been designed for double-sided printing. Hard copies supplied by Biota are printed on recycled paper. Cube:Current:503 (Kimberley Hub Wet Season):Doc:Flora:503 flora v7_2.doc 3 A Vegetation and Flora Survey of James Price Point: Wet Season 2009 4 Cube:Current:503 (Kimberley Hub Wet Season):Doc:Flora:503 flora v7_2.doc Biota A Vegetation and Flora Survey of James Price Point: Wet Season 2009 A Vegetation and Flora Survey of James Price -

Plant Associations and Descriptions for American Memorial Park, Commonwealth of the Northern Mariana Islands, Saipan
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Vegetation Inventory Project American Memorial Park Natural Resource Report NPS/PACN/NRR—2013/744 ON THE COVER Coastal shoreline at American Memorial Park Photograph by: David Benitez Vegetation Inventory Project American Memorial Park Natural Resource Report NPS/PACN/NRR—2013/744 Dan Cogan1, Gwen Kittel2, Meagan Selvig3, Alison Ainsworth4, David Benitez5 1Cogan Technology, Inc. 21 Valley Road Galena, IL 61036 2NatureServe 2108 55th Street, Suite 220 Boulder, CO 80301 3Hawaii-Pacific Islands Cooperative Ecosystem Studies Unit (HPI-CESU) University of Hawaii at Hilo 200 W. Kawili St. Hilo, HI 96720 4National Park Service Pacific Island Network – Inventory and Monitoring PO Box 52 Hawaii National Park, HI 96718 5National Park Service Hawaii Volcanoes National Park – Resources Management PO Box 52 Hawaii National Park, HI 96718 December 2013 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics. These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate high-priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -

Guidelines for Using the Checklist
Guidelines for using the checklist Cymbopogon excavatus (Hochst.) Stapf ex Burtt Davy N 9900720 Synonyms: Andropogon excavatus Hochst. 47 Common names: Breëblaarterpentyngras A; Broad-leaved turpentine grass E; Breitblättriges Pfeffergras G; dukwa, heng’ge, kamakama (-si) J Life form: perennial Abundance: uncommon to locally common Habitat: various Distribution: southern Africa Notes: said to smell of turpentine hence common name E2 Uses: used as a thatching grass E3 Cited specimen: Giess 3152 Reference: 37; 47 Botanical Name: The grasses are arranged in alphabetical or- Rukwangali R der according to the currently accepted botanical names. This Shishambyu Sh publication updates the list in Craven (1999). Silozi L Thimbukushu T Status: The following icons indicate the present known status of the grass in Namibia: Life form: This indicates if the plant is generally an annual or G Endemic—occurs only within the political boundaries of perennial and in certain cases whether the plant occurs in water Namibia. as a hydrophyte. = Near endemic—occurs in Namibia and immediate sur- rounding areas in neighbouring countries. Abundance: The frequency of occurrence according to her- N Endemic to southern Africa—occurs more widely within barium holdings of specimens at WIND and PRE is indicated political boundaries of southern Africa. here. 7 Naturalised—not indigenous, but growing naturally. < Cultivated. Habitat: The general environment in which the grasses are % Escapee—a grass that is not indigenous to Namibia and found, is indicated here according to Namibian records. This grows naturally under favourable conditions, but there are should be considered preliminary information because much usually only a few isolated individuals. -

Chimanimani National Reserve, Which Forms the Core Zone of the Conservation Area in Mozambique (634 Km²)
CCCHHHIIIMMMAAANNNIIIMMMAAANNNIII NNNAAATTTIIIOOONNNAAALLL RRREEESSSEEERRRVVVEEE MMAANNAAGGEEMMEENNTT PPLLAANN VVOOLLUUMMEE IIII 2010 Prepared by: Andrea Ghiurghi, Stefaan Dondeyne and James Hugh Bannerman AGRICONSULTING S.p.A. RNC Management Plan 2010 Volume 2 2 Index to Volume 2 1 KEY ACTIONS TO BE IMPLEMENTED DURING THE FIRST TWO YEARS OF MANAGEMENT ................................................................................................................................................. 7 2 PROGRAMME 1 – REVISION OF LIMITS, STRATEGY TO DEAL WITH PEOPLE INSIDE THE RESERVE, ZONING, AND ADMINISTRATIVE STRUCTURE ...................................................... 11 2.1 REVISION OF THE BOUNDARIES .......................................................................................................... 11 2.1.1 Introduction.................................................................................................................................. 11 2.1.2 Guiding principles........................................................................................................................ 11 2.1.3 Objective ...................................................................................................................................... 12 2.1.4 Adjustment of the Reserve Boundaries......................................................................................... 12 2.1.5 Strategy to deal with people living inside the Reserve ................................................................. 13 2.1.6 Adjustement -

(Poaceae: Andropogoneae): a Presumed Extinct Grass from Andhra Pradesh, India
Phytotaxa 497 (2): 147–156 ISSN 1179-3155 (print edition) https://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2021 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.497.2.7 Rediscovery of Parahyparrhenia bellariensis (Poaceae: Andropogoneae): A presumed extinct grass from Andhra Pradesh, India SHAHID NAWAZ LANDGE1* & RAJENDRA D. SHINDE2 1 The Blatter Herbarium (BLAT), St. Xavier’s College (Autonomous), Mumbai 400001. �[email protected]; https://orcid.org/0000-0002-6734-6749 2 St. Xavier’s College (Autonomous), Mumbai 400001. �[email protected]; https://orcid.org/0000-0002-9315-9883 *Corresponding author: �[email protected] Abstract Parahyparrhenia bellariensis, an extremely rare and highly narrow endemic grass, has been rediscovered after almost 184 years from Cuddapah [Kadapa] district, Andhra Pradesh. The first description of its complete habit, basal portion and other features of the spikelets are provided along with new locality of its occurrence. In addition, photographs of the habitats, live plants, and a key to distinguish two Indian endemic species, distribution map and illustration are provided. As per the IUCN Red List Criteria this species is assessed here as Critically Endangered (CR). In order to facilitate the prospective conservation of this grass, we have discussed about the peculiarity of its habitat. Keywords: Eastern Ghats, Endemic, Gandikota Fort Hill, Gooty fort hill, Peninsular India, Robert Wight Introduction Parahyparrhenia A. Camus (1950: 404) is a fairly small, Afro-Eurasian genus, belonging to the tribe Andropogoneae Dumortier (1824: 84). It comprises seven species distributed in Africa and Tropical Asia, of which two species, namely, P. bellariensis (Hackel 1885: 123) Clayton (1972: 448) and recently described P.