Plant Species and Functional Diversity Along Altitudinal Gradients, Southwest Ethiopian Highlands
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Regional Sources of Precipitation in the Ethiopian Highlands Regionala Källor Till Nederbörden I Det Etiopiska Höglandet
Independent Project at the Department of Earth Sciences Självständigt arbete vid Institutionen för geovetenskaper 2015: 2 Regional Sources of Precipitation in the Ethiopian Highlands Regionala källor till nederbörden i det Etiopiska höglandet Elnaz Ashkriz DEPARTMENT OF EARTH SCIENCES INSTITUTIONEN FÖR GEOVETENSKAPER Independent Project at the Department of Earth Sciences Självständigt arbete vid Institutionen för geovetenskaper 2015: 2 Regional Sources of Precipitation in the Ethiopian Highlands Regionala källor till nederbörden i det Etiopiska höglandet Elnaz Ashkriz Copyright © Elnaz Ashkriz and the Department of Earth Sciences, Uppsala University Published at Department of Earth Sciences, Uppsala University (www.geo.uu.se), Uppsala, 2015 Sammanfattning Regionala källor till nederbörden i det Etiopiska höglandet Elnaz Ashkriz Denna uppsats undersöker ursprunget till den stora mängd nederbörd som faller i det etiopiska höglandet. Med Moisture transport into the Ethiopian Highlands av Ellen Viste och Asgeir Sorteberg (2011) som grund syftar denna uppsats till att jämföra samma data men genom att titta på ett mycket kortare intervall för att se vad som försummas när undersökningar på större skalor utförs. Medan undersökningen av Viste och Sorteberg (2011) fokuserar på de två regnrikaste månaderna, juli och augusti under elva år, 1998-2008, så fokuserar denna uppsats enbart på juli år 2008. Syftet med denna uppsats var att se vart nederbörden till det Etiopiska höglandet kommer ifrån under juli månad 2008. För att undersöka detta så har man valt att titta på parametrar såsom horisontell- och vertikal vindriktning på olika höjder samt fukt- innehållet i dessa vindar. Som grund för undersökningen så har denna uppsats, likt Vistes och Sortebergs, använt ERA-Interim data. -
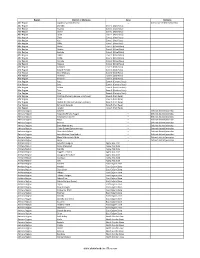
Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

One for All - Artemisia Absinthium (Afsanteen) “A Potent Unani Drug”
Review article One for All - Artemisia absinthium (Afsanteen) “A Potent Unani Drug” Ayshah Hashimi1, Mantasha Binth Siraj2, Yasmeen Ahmed3, Md. Akhtar Siddiqui4, Umar Jahangir5* 1,2,3 M.D. Scholar, 4 Professor, 5 Assistant Professor Department of Moalajat, School of Unani Medical Education and Research Jamia Hamdard, New Delhi, India ABSTRACT The therapeutic use of the wormwood plant Artemisia absinthium L. dates back to at least Roman times. There are more than 200 plants in the genus Artemisia- including southern wormwood, petite wormwood and Grande wormwood and encompasses about 500 species. The best-known species of wormwood is Artemisia absinthium, native to temperate Eurasia and North Africa and is branded for its extreme bitterness. It is a magical greens booze used as carminative to support healthy appetite, balances healthy flora, cleanse the digestive tract of parasite and toxins. It possesses anti-inflammatory, immunomodulatory, hepatoprotective, anti-helminthic and anti-depressant activity. Thujone excites nervous system when taken in small amount. Due to contrary history of wormwood, its application in individuals should be preceded by a thorough and cautious risk-benefit analysis. In this appraisal an attempt is done to validate scientifically, mentioned therapeutic potential of Artemisia absinthium in classical Unani literature using PubMed, Science Direct researches. Keywords Afsanteen, Wormwood, Thujone, Unani, Hepatoprotective INTRODUCTION largest and highly advanced family with approximately 1528 (Mukherjee, 2006), (TYROCITY, 2018) or 1620 (Petruzzello, Healing with medicinal plants is as old as mankind itself. 2018), (Panero, et al., 2012) genera and 22750 or 23600 Since prehistoric times, in quest for rescue for their disease, the species of herbs, shrubs and trees. -

Genetic Adaptation to High Altitude in the Ethiopian Highlands
Scheinfeldt et al. Genome Biology 2012, 13:R1 http://genomebiology.com/2012/13/1/R1 RESEARCH Open Access Genetic adaptation to high altitude in the Ethiopian highlands Laura B Scheinfeldt1, Sameer Soi1, Simon Thompson1, Alessia Ranciaro1, Dawit Woldemeskel2, William Beggs1, Charla Lambert1,3, Joseph P Jarvis1, Dawit Abate2, Gurja Belay2 and Sarah A Tishkoff1,4* Abstract Background: Genomic analysis of high-altitude populations residing in the Andes and Tibet has revealed several candidate loci for involvement in high-altitude adaptation, a subset of which have also been shown to be associated with hemoglobin levels, including EPAS1, EGLN1, and PPARA, which play a role in the HIF-1 pathway. Here, we have extended this work to high- and low-altitude populations living in Ethiopia, for which we have measured hemoglobin levels. We genotyped the Illumina 1M SNP array and employed several genome-wide scans for selection and targeted association with hemoglobin levels to identify genes that play a role in adaptation to high altitude. Results: We have identified a set of candidate genes for positive selection in our high-altitude population sample, demonstrated significantly different hemoglobin levels between high- and low-altitude Ethiopians and have identified a subset of candidate genes for selection, several of which also show suggestive associations with hemoglobin levels. Conclusions: We highlight several candidate genes for involvement in high-altitude adaptation in Ethiopia, including CBARA1, VAV3, ARNT2 and THRB. Although most of these genes have not been identified in previous studies of high-altitude Tibetan or Andean population samples, two of these genes (THRB and ARNT2) play a role in the HIF-1 pathway, a pathway implicated in previous work reported in Tibetan and Andean studies. -

MUST KNOW Geography
AP World History Ms. Avar File: Geography MUST KNOW Geography Description You must understand Geography to effectively study world history. Practice and learn the skills in your Geography 101 packet (given to you the first week of school), know the location of world regions and sub regions and be able to identify and locate key nations, landforms and bodies of water listed on this sheet. POLTICAL MAPS Instructions: Neatly locate, outline in color and label ALL of the following countries on your Continent Political maps. Use the world map at end of your textbook, Google Maps and/or worldatlas.com (search by continent) AFRICA North Africa Algeria Egypt East Ethiopia Kenya Libya Morocco Africa Madagascar Somalia Tunisia Sudan Tanzania West Africa Chad Benin Ghana Equatorial Cameroon Rwanda Mali Mauritania Senegal Africa Uganda Sudan Niger Nigeria Central African Republic Togo Cote D’Ivoire Democratic Republic of the Congo Southern Africa Angola Botswana Zimbabwe Zambia Republic of South Africa Mozambique ASIA East Asia Japan China SE Asia Cambodia Indonesia Vietnam North Korea South Korea Myanmar (Burma) Malaysia Thailand Taiwan Mongolia Philippines Singapore Laos South Asia Afghanistan Bangladesh SW Asia / Iran Iraq Turkey India Pakistan Middle East Jordan Israel Nepal Syria Saudi Arabia Central Asia Kazakhstan EUROPE Western France Germany Ireland Eastern Hungary Poland Europe Portugal Spain Switzerland Europe Romania Russia England/Great Britain/United Kingdom “U.K.” Ukraine Serbia Austria Czech Republic Northern Finland Norway Southern -

Food Supply Prospects - 2009
FOOD SUPPLY PROSPECTS - 2009 Disaster Management and Food Security Sector (DMFSS) Ministry of Agriculture and Rural Development (MoARD) Addis Ababa Ethiopia February 10, 2009 TABLE OF CONTENTS Pages LIST OF GLOSSARY OF LOCAL NAMES 2 ACRONYMS 3 EXECUTIVE SUMMARY 5 - 8 INTRODUCTION 9 - 12 REGIONAL SUMMARY 1. SOMALI 13 - 17 2. AMHARA 18 – 22 3. SNNPR 23 – 28 4. OROMIYA 29 – 32 5. TIGRAY 33 – 36 6. AFAR 37 – 40 7. BENSHANGUL GUMUZ 41 – 42 8. GAMBELLA 43 - 44 9. DIRE DAWA ADMINISTRATIVE COUNSEL 44 – 46 10. HARARI 47 - 48 ANNEX – 1 NEEDY POPULATION AND FOOD REQUIREMENT BY WOREDA 2 Glossary Azmera Rains from early March to early June (Tigray) Belg Short rainy season from February/March to June/July (National) Birkads cemented water reservoir Chat Mildly narcotic shrub grown as cash crop Dega Highlands (altitude>2500 meters) Deyr Short rains from October to November (Somali Region) Ellas Traditional deep wells Enset False Banana Plant Gena Belg season during February to May (Borena and Guji zones) Gu Main rains from March to June ( Somali Region) Haga Dry season from mid July to end of September (Southern zone of of Somali ) Hagaya Short rains from October to November (Borena/Bale) Jilal Long dry season from January to March ( Somali Region) Karan Rains from mid-July to September in the Northern zones of Somali region ( Jijiga and Shinile zones) Karma Main rains fro July to September (Afar) Kolla Lowlands (altitude <1500meters) Meher/Kiremt Main rainy season from June to September in crop dependent areas Sugum Short rains ( not more than 5 days -

Behavior of Various Types of Seeds of Two Species of Yams Tuber (Dioscorea Cayenensis Lam
International Research Journal of Agricultural Science and Soil Science (ISSN: 2251-0044) Vol. 5(2) pp. 58-66, February 2015 DOI: http:/dx.doi.org/10.14303/irjas.2015.025 Available online http://www.interesjournals.org/IRJAS Copyright © 2015 International Research Journals Full Length Research Paper Behavior of various types of seeds of two species of yams tuber (Dioscorea cayenensis Lam. and Dioscorea rotundata Poir.) in Gabon *Ondo Ovono Paul1, Kevers Claire2, Dommes Jacques2 1Unit of Agrobiology Research, Higher National Institute of Agronomy and Biotechnology, Université des Sciences et Techniques de Masuku, B.P. 941, Masuku, Gabon. 2Plant Molecular Biology and Biotechnology Unit, B-22, University of Liège, Sart Tilman B 22, 4000 Liège, Belgium. Corresponding author’s E-mail: [email protected] Abstract Low multiplication ratio of yam and scarcity of planting materials are major constraints militating against sustainable yam production. In order to evaluate the behavior of the four various types of seeds of two species of yams Dioscorea cayenensis and Dioscorea rotundata, cultivated on the experimental ground of the Higher National Institute of Agronomy and Biotechnology (INSAB), a test was realized in a randomized complete block design with six replications. The samples were cut and three levels of each tuber were used: proximal, medial and distal parts of the tuber. The fragments of tuber and the whole tuber represent the various types of seed used in this work. The results showed significant (P<0.05) differences in number of plants emerged and time of emergence in a mixture of 40% soil and 60% sand three months and half after planting. -

Hygienic Practice Among Milk and Cottage Cheese Handlers in Districts of Gamo and Gofa Zone, Southern Ethiopia
Research Article Volume 12:2, 2021 Journal of Veterinary Science & Technology ISSN: 2157-7579 Open Access Knowledge; Hygienic Practice among Milk and Cottage Cheese Handlers in Districts of Gamo and Gofa Zone, Southern Ethiopia Edget Alembo* Department of Animal Science, Arba Minch University, Arba Minch, Ethiopia Abstract A cross-sectional questionnaire survey was conducted in Arba Minch Zuria and Demba Gofa districts of Gamo and Gofa Zone of the Southern nation nationalities and people’s regional state with the objectives of assessing knowledge of hygienic practice of milk and cheese handlers in both study area. For this a total of 102 farmers who involved in milking, collecting and retailing of milk were included in the study area. Data obtained from questionnaire survey were analyzed by descriptive statistics and Chi –square test, using the Statistical package for social science (SPSS Version 17). The participants of this study were woman of different age group and 27(52.9%) of participants in Arba Minch Zuria and 32(64.7%) in Demba Gofa were >36 years old. The majority of participants 21(41.2%) and 22(43.1%) were educated up to grade 1-8 in Arba Minch Zuria and Demba Gofa, respectively. This had an impact on hygienic practice of milking and milk handling. The difference in hygienic handling, training obtained and cheese making practice among the study areas were statistically significant (p<0.05). There was also a statistically significant difference in hand washing and utensil as well as manner of washing between the two study areas (p<0.01). Finally this study revealed that there were no variation in Antibiotic usage and Practice of treating sick animal in both study area (p>0.05) with significant difference in Prognosis, Level of skin infection and Selling practice among study participants in both study areas (p<0.05). -

Conservation of Greater Sage-Grouse
#714 CHAPTER TWENTY-FOUR Conservation of Greater Sage-Grouse A SYNTHESIS OF CURRENT TRENDS AND FUTURE MANAGEMENT J. W. Connelly, S. T. Knick, C. E. Braun, W. L. Baker, E. A. Beever, T. Christiansen, K. E. Doherty, E. O. Garton, S. E. Hanser, D. H. Johnson, M. Leu, R. F. Miller, D. E. Naugle, S. J. Oyler-McCance, D. A. Pyke, K. P. Reese, M. A. Schroeder, S. J. Stiver, B. L. Walker, and M. J. Wisdom Abstract. Recent analyses of Greater Sage-Grouse very low densities in some areas, coupled with (Centrocercus urophasianus) populations indicate large areas of important sagebrush habitat that are substantial declines in many areas but relatively relatively unaffected by the human footprint, sug- stable populations in other portions of the species’ gest that Greater Sage-Grouse populations may be range. Sagebrush (Artemisia spp.) habitats neces- able to persist into the future. We summarize the sary to support sage-grouse are being burned by status of sage-grouse populations and habitats, large wildfires, invaded by nonnative plants, and provide a synthesis of major threats and chal- developed for energy resources (gas, oil, and lenges to conservation of sage-grouse, and suggest wind). Management on public lands, which con- a roadmap to attaining conservation goals. tain 70% of sagebrush habitats, has changed over the last 30 years from large sagebrush control Key Words: Centrocercus urophasianus, Greater projects directed at enhancing livestock grazing to Sage-Grouse, habitats, management, populations, a greater emphasis on projects that often attempt restoration, sagebrush. to improve or restore ecological integrity. Never- theless, the mandate to manage public lands to Conservación del Greater Sage-Grouse: provide traditional consumptive uses as well as Una Síntesis de las Tendencias Actuales y del recreation and wilderness values is not likely to Manejo Futuro change in the near future. -

Etude Florisitique D'une Végétation Naturelle En Anthropise: Cas De La
UNIVERSITE DE KISANGANI CENTRE UNIVERSITAIRE EXTENSION DE BUKAVU C.U.B B.P. 570 BUKAVU FACULTE DES SCIENCES ETUDE FLORISTIQUE D’UNE VEGETATION NATURELLE EN MILIEU ANTHROPISE : CAS DE LA FORMATION ARBUSTIVE XEROPHILE DE CIBINDA, AU NORD DE BUKAVU Par Chantal KABOYI Nzabandora Mémoire présenté et défendu en vue de L’obtention du grade de Licence en Sciences Option : Biologie Orientation : Phytosociologie et Taxonomie végétale Directeur : Prof. Dr Jean-Baptiste Dhetchuvi Matchu-Mandje Année académique 2003-2004 II DEDICACE A nos très chers parents, Joseph NZABANDORA et Florence KOFIMOJA, pour tant d’amour et de sacrifice consentis dans notre parcours terrestre et dont l’aboutissement de nos études universitaires demeure un des témoignages les plus éloquents que nous n’ayons jamais eu dans la vie ; A notre charmante sœur jumelle Julienne BASEKE avec qui, de par notre existence, nous avons été faites pour partager une vie inséparable et chaleureuse ; A nos petits frères et sœurs, pour tant d’amour et de respect qu’ils n’on cessé de témoigner à notre égard, que ce travail soit pour vous un exemple à suivre ; A notre futur époux et nos futurs enfants pour l’amour, l’attente et la compréhension qui nous caractériseront toujours. III AVANT-PROPOS Au terme de notre parcours universitaire, il nous est un agréable devoir de formuler nos vifs remerciements à tous ceux qui, de près ou de loin, ont contribué à notre formation tant morale qu'intellectuelle. Nos sincères remerciements s'adressent, tout d'abord, aux autorités académiques, administratives ainsi qu'aux professeurs, chefs de travaux et assistants du Centre Universitaire Extension de Bukavu (CUB), pour toutes les théories apprises tout au long de notre séjour en son sein. -

(Poaceae: Andropogoneae): a Presumed Extinct Grass from Andhra Pradesh, India
Phytotaxa 497 (2): 147–156 ISSN 1179-3155 (print edition) https://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2021 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.497.2.7 Rediscovery of Parahyparrhenia bellariensis (Poaceae: Andropogoneae): A presumed extinct grass from Andhra Pradesh, India SHAHID NAWAZ LANDGE1* & RAJENDRA D. SHINDE2 1 The Blatter Herbarium (BLAT), St. Xavier’s College (Autonomous), Mumbai 400001. �[email protected]; https://orcid.org/0000-0002-6734-6749 2 St. Xavier’s College (Autonomous), Mumbai 400001. �[email protected]; https://orcid.org/0000-0002-9315-9883 *Corresponding author: �[email protected] Abstract Parahyparrhenia bellariensis, an extremely rare and highly narrow endemic grass, has been rediscovered after almost 184 years from Cuddapah [Kadapa] district, Andhra Pradesh. The first description of its complete habit, basal portion and other features of the spikelets are provided along with new locality of its occurrence. In addition, photographs of the habitats, live plants, and a key to distinguish two Indian endemic species, distribution map and illustration are provided. As per the IUCN Red List Criteria this species is assessed here as Critically Endangered (CR). In order to facilitate the prospective conservation of this grass, we have discussed about the peculiarity of its habitat. Keywords: Eastern Ghats, Endemic, Gandikota Fort Hill, Gooty fort hill, Peninsular India, Robert Wight Introduction Parahyparrhenia A. Camus (1950: 404) is a fairly small, Afro-Eurasian genus, belonging to the tribe Andropogoneae Dumortier (1824: 84). It comprises seven species distributed in Africa and Tropical Asia, of which two species, namely, P. bellariensis (Hackel 1885: 123) Clayton (1972: 448) and recently described P. -

Adaptation of Plants Derived from Cultivated Yam of Dioscorea Cayenensis–D
Adaptation of plants derived from cultivated yam of Dioscorea cayenensis–D. rotundata complex species seeds germination in two agroecological zones of Benin Assaba Idossou Elie 1, *, Yolou Mounirou 1, Bello Saliou 3, Babalakoun Adonis 1, Tiama Djakaridja 2 and Zoundjihekpon Jeanne 1 1 Laboratory of Ecological Genetics, Department of Genetics and Biotechnologies, Faculty of Science and Technology, University of Abomey–Calavi, (LGE/FAST/UAC), BP 4521 Cotonou, Republic of Benin. 2 Biosciences Laboratory, Genetics and Plant Improvement Team, UFR / SVT, Joseph KI ZERBO University, Ouagadougou, Burkina Faso. 3 Agricultural Research Center of South-Benin, National Agricultural Research Institute of Benin (CRA-Sud/ INRAB). World Journal of Advanced Research and Reviews, 2021, 09(03), 027–041 Publication history: Received on 06 January 2021; revised on 15 February 2021; accepted on 17 February 2021 Article DOI: https://doi.org/10.30574/wjarr.2021.9.3.0019 Abstract Vegetative propagation of plants promotes the accumulation of viruses in plant material; this causes the loss of vigor and consequently the drop of vegetable yield. Keep up productivity level of vegetatively propagated plants; it is therefore important to regenerate genetic material by sexual reproduction to improve the biodiversity. The main objective of this study is to improve yam seeds by sexual way, specifically to assess the response of seedlings from yam seeds in two agroecological areas, area IV (Djougou), in Sudanese climate and area V (Bantè), in transition climate. Seedling were transplanted in this agroecological areas using a completely Randomized design with three replications. Data was analyzed using one way ANOVA at 5% level of significance and a mean comparison test.