A Heterodimeric DNA Polymerase: Evidence That Members Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
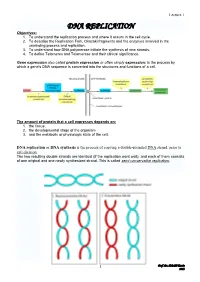
DNA REPLICATION Objectives: 1
Lecture 1 DNA REPLICATION Objectives: 1. To understand the replication process and where it occurs in the cell cycle. 2. To describe the Replication Fork, Okazaki fragments and the enzymes involved in the unwinding process and replication. 3. To understand how DNA polymerase initiate the synthesis of new strands. 4. To define Telomeres and Telomerase and their clinical significance. Gene expression also called protein expression or often simply expression: is the process by which a gene's DNA sequence is converted into the structures and functions of a cell. The amount of protein that a cell expresses depends on: 1. the tissue, 2. the developmental stage of the organism 3. and the metabolic or physiologic state of the cell. DNA replication or DNA synthesis is the process of copying a double-stranded DNA strand, prior to cell division. The two resulting double strands are identical (if the replication went well), and each of them consists of one original and one newly synthesized strand. This is called semi conservative replication. 1 Prof. Dr. H.D.El-Yassin 2013 Lecture 1 The process of replication consists of three steps, initiation, replication and termination. 1. Prokaryotic replication Basic Requirement for DNA Synthesis 1. Substrates: the four deoxy nucleosides triphosphates are needed as substrates for DNA synthesis. Cleavage of the high-energy phosphate bond between the α and β phosphates provides the energy for the addition of the nucleotide. 2. Template: DNA replication cannot occur without a template. A template is required to direct the addition of the appropriate complementary deoxynucleotide to the newly synthesized DNA strand. -
DNA Polymerase Exchange and Lesion Bypass in Escherichia Coli
DNA Polymerase Exchange and Lesion Bypass in Escherichia Coli The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kath, James Evon. 2016. DNA Polymerase Exchange and Lesion Bypass in Escherichia Coli. Doctoral dissertation, Harvard University, Graduate School of Arts & Sciences. Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:26718716 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ! ! ! ! ! ! ! DNA!polymerase!exchange!and!lesion!bypass!in!Escherichia)coli! ! A!dissertation!presented! by! James!Evon!Kath! to! The!Committee!on!Higher!Degrees!in!Biophysics! ! in!partial!fulfillment!of!the!requirements! for!the!degree!of! Doctor!of!Philosophy! in!the!subject!of! Biophysics! ! Harvard!University! Cambridge,!Massachusetts! October!2015! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ©!2015!L!James!E.!Kath.!Some!Rights!Reserved.! ! This!work!is!licensed!under!the!Creative!Commons!Attribution!3.0!United!States!License.!To! view!a!copy!of!this!license,!visit:!http://creativecommons.org/licenses/By/3.0/us! ! ! Dissertation!Advisor:!Professor!Joseph!J.!Loparo! ! ! !!!!!!!!James!Evon!Kath! ! DNA$polymerase$exchange$and$lesion$bypass$in$Escherichia)coli$ $ Abstract$ ! Translesion! synthesis! (TLS)! alleviates! -

A Proposal to Rename the Hyperthermophile Pyrococcus Woesei As Pyrococcus Furiosus Subsp
Archaea 1, 277–283 © 2004 Heron Publishing—Victoria, Canada A proposal to rename the hyperthermophile Pyrococcus woesei as Pyrococcus furiosus subsp. woesei WIROJNE KANOKSILAPATHAM,1 JUAN M. GONZÁLEZ,1,2 DENNIS L. MAEDER,1 1,3 1,4 JOCELYNE DIRUGGIERO and FRANK T. ROBB 1 Center of Marine Biotechnology, University of Maryland Biotechnology Institute, Baltimore, MD 21202, USA 2 Present address: IRNAS-CSIC, P.O. Box 1052, 41080 Sevilla, Spain 3 Department of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20274, USA 4 Corresponding author ([email protected]) Received April 8, 2004; accepted July 28, 2004; published online August 31, 2004 Summary Pyrococcus species are hyperthermophilic mem- relatively rapidly at temperatures above 90 °C and produce ° bers of the order Thermococcales, with optimal growth tem- H2S from elemental sulfur (S ) (Zillig et al. 1987). Two Pyro- peratures approaching 100 °C. All species grow heterotrophic- coccus species, P. furiosus (Fiala and Stetter 1986) and ° ally and produce H2 or, in the presence of elemental sulfur (S ), P. woesei (Zillig et al. 1987), were isolated from marine sedi- H2S. Pyrococcus woesei and P.furiosus were isolated from ma- ments at a Vulcano Island beach site in Italy. They have similar rine sediments at the same Vulcano Island beach site and share morphological and physiological characteristics: both species many morphological and physiological characteristics. We re- are cocci and move by means of a tuft of polar flagella. They port here that the rDNA operons of these strains have identical are heterotrophic and grow optimally at 95–100 °C, utilizing sequences, including their intergenic spacer regions and part of peptides as major carbon and nitrogen sources. -

Conversion of OX174 and Fd Single-Stranded DNA to Replicative Forms in Extracts of Escherichia Coli (Dnac, Dnad, and Dnag Gene Products/DNA Polymerase III) REED B
Proc. Nat. Acad. Sci. USA Vol. 69, No. 11, pp. 3233-3237, November 1972 Conversion of OX174 and fd Single-Stranded DNA to Replicative Forms in Extracts of Escherichia coli (dnaC, dnaD, and dnaG gene products/DNA polymerase III) REED B. WICKNER, MICHEL WRIGHT, SUE WICKNER, AND JERARD HURWITZ Department of Developmental Biology and Cancer, Division of Biological Sciences, Albert Einstein College of Medicine, Bronx, New York 10461 Communicated by Harry Eagle, August 28, 1972 ABSTRACT 4X174 and M13 (fd) single-stranded cir- MATERIALS AND METHODS cular DNAs are converted to their replicative forms by ex- tracts of E. coli pol Al cells. We find that the qX174 DNA- [a-32P]dTTP was obtained from New England Nuclear Corp. dependent reaction requires Mg++, ATP, and all four de- OX174 DNA was prepared by the method of Sinsheimer (7) oxynucleoside triphosphates, but not CTP, UTP, or GTP. or Franke and Ray (8), while fd viral DNA was prepared as This reaction also involves the products of the dnaC, dnaD, dnaE (DNA polymerase III), and dnaG genes, described (9). Pancreatic RNase was the highest grade ob- but not that of dnaF (ribonucleotide reductase). The in tainable from Worthington Biochemical Corp. It was further vitro conversion of fd single-stranded DNA to the replica- freed of possible contaminating DNase by heating a solution tive form requires all four ribonucleoside triphosphates, (2 mg/ml in 15 mM sodium citrate, pH 5) at 800 for 10 min. Mg++, and all four deoxynucleoside triphosphates. The E. protein was purified from E. coli strain reaction involves the product of gene dnaE but not those coli unwinding of genes dnaC, dnaD, dnaF, or dnaG. -

Product Sheet Info
Master Clone List for NR-19279 ® Vibrio cholerae Gateway Clone Set, Recombinant in Escherichia coli, Plates 1-46 Catalog No. NR-19279 Table 1: Vibrio cholerae Gateway® Clones, Plate 1 (NR-19679) Clone ID Well ORF Locus ID Symbol Product Accession Position Length Number 174071 A02 367 VC2271 ribD riboflavin-specific deaminase NP_231902.1 174346 A03 336 VC1877 lpxK tetraacyldisaccharide 4`-kinase NP_231511.1 174354 A04 342 VC0953 holA DNA polymerase III, delta subunit NP_230600.1 174115 A05 388 VC2085 sucC succinyl-CoA synthase, beta subunit NP_231717.1 174310 A06 506 VC2400 murC UDP-N-acetylmuramate--alanine ligase NP_232030.1 174523 A07 132 VC0644 rbfA ribosome-binding factor A NP_230293.2 174632 A08 322 VC0681 ribF riboflavin kinase-FMN adenylyltransferase NP_230330.1 174930 A09 433 VC0720 phoR histidine protein kinase PhoR NP_230369.1 174953 A10 206 VC1178 conserved hypothetical protein NP_230823.1 174976 A11 213 VC2358 hypothetical protein NP_231988.1 174898 A12 369 VC0154 trmA tRNA (uracil-5-)-methyltransferase NP_229811.1 174059 B01 73 VC2098 hypothetical protein NP_231730.1 174075 B02 82 VC0561 rpsP ribosomal protein S16 NP_230212.1 174087 B03 378 VC1843 cydB-1 cytochrome d ubiquinol oxidase, subunit II NP_231477.1 174099 B04 383 VC1798 eha eha protein NP_231433.1 174294 B05 494 VC0763 GTP-binding protein NP_230412.1 174311 B06 314 VC2183 prsA ribose-phosphate pyrophosphokinase NP_231814.1 174603 B07 108 VC0675 thyA thymidylate synthase NP_230324.1 174474 B08 466 VC1297 asnS asparaginyl-tRNA synthetase NP_230942.2 174933 B09 198 -

Evidence for Associated Horizontal Gene Transfer in Pyrococcus Furiosus
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Digital.CSIC J Appl Genet 50(4), 2009, pp. 421–430 Original article CRISPR elements in the Thermococcales: evidence for associated horizontal gene transfer in Pyrococcus furiosus M.C. Portillo, J.M. Gonzalez Institute for Natural Resources and Agrobiology, IRNAS-CSIC, Sevilla, Spain Abstract. The presence and distribution of CRISPR (clustered regularly interspaced short palindrome repeat)el- ements in the archaeal order Thermococcales were analyzed. Four complete genome sequences from the species Pyrococcus abyssi, P. furiosus, P. horikoshii, and Thermococcus kodakaraensis were studied. A fragment of the genome of P. furiosus was flanked by CRISPR elements upstream and by a single element downstream. The composition of the gene sequences contained in this genome fragment (positions 699013 to 855319) showed sig- nificant differences from the other genes in the P. furiosus genome. Differences were observed in the GC content at the third codon positions and the frequency of codon usage between the genes located in the analyzed fragment and the other genes in the P. furiosus genome. These results represent the first evidence suggesting that repeated CRISPR elements can be involved in horizontal gene transfer and genomic differentiation of hyperthermophilic Archaea. Keywords: CRISPR, gene transfer, genome, hyperthermophilic Archaea, Pyrococcus furiosus. Introduction spacers and repetitive elements modified the phage-resistance phenotype of bacteria Genomic regions consisting of tandem repeat (Barrangou et al. 2007). The incorporation of short DNA elements, typically of 21–47 base pairs (bp) fragments of foreign DNA into the genome of in length, separated by nonrepetitive spacer se- prokaryotes at CRISPR loci has been reported quences of approximately similar length, have (Bolotin et al. -

Roles of DNA Polymerases V and II in SOS-Induced Error-Prone and Error-Free Repair in Escherichia Coli
Colloquium Roles of DNA polymerases V and II in SOS-induced error-prone and error-free repair in Escherichia coli Phuong Pham*, Savithri Rangarajan*, Roger Woodgate†, and Myron F. Goodman*‡ *Departments of Biological Sciences and Chemistry, Hedco Molecular Biology Laboratories, University of Southern California, Los Angeles, CA 90089-1340; and †Section on DNA Replication, Repair and Mutagenesis, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892-2725 DNA polymerase V, composed of a heterotrimer of the DNA regulated at the transcriptional level by the LexA protein (14). damage-inducible UmuC and UmuD2 proteins, working in conjunc- Many of these genes encode proteins required to repair DNA tion with RecA, single-stranded DNA (ssDNA)-binding protein damage (15). The overriding importance of DNA repair is (SSB),  sliding clamp, and ␥ clamp loading complex, are respon- apparent from the observation that a single pyrimidine dimer is sible for most SOS lesion-targeted mutations in Escherichia coli,by lethal in E. coli strains defective for excision and recombinational catalyzing translesion synthesis (TLS). DNA polymerase II, the repair (16). These experiments were among the first to demon- product of the damage-inducible polB (dinA ) gene plays a pivotal strate the essential contribution of DNA repair to cell survival. role in replication-restart, a process that bypasses DNA damage in Excision and recombination repair pathways are referred to as an error-free manner. Replication-restart takes place almost im- ‘‘error-free’’ because they do not result in an increase in muta- mediately after the DNA is damaged (Ϸ2 min post-UV irradiation), tion rate above spontaneous background levels (1). -

Gene Cloning and Characterization of NADH Oxidase from Thermococcus Kodakarensis
African Journal of Biotechnology Vol. 10(78), pp. 17916-17924, 7 December, 2011 Available online at http://www.academicjournals.org/AJB DOI: 10.5897/AJB11.989 ISSN 1684–5315 © 2011 Academic Journals Full Length Research Paper Gene cloning and characterization of NADH oxidase from Thermococcus kodakarensis Naeem Rashid 1*, Saira Hameed 2, Masood Ahmed Siddiqui 3 and Ikram-ul-Haq 2 1School of Biological Sciences, University of the Punjab, Quaid-e-Azam Campus, Lahore 54590, Pakistan. 2Institute of Industrial Biotechnology, GC University, Lahore, Pakistan. 3Department of Chemistry, University of Balochistan, Quetta, Pakistan. Accepted 12 October, 2011 The genome search of Thermococcus kodakarensis revealed three open reading frames, Tk0304, Tk1299 and Tk1392 annotated as nicotinamide adenine dinucleotide (NADH) oxidases. This study deals with cloning, and characterization of Tk0304. The gene, composed of 1320 nucleotides, encodes a protein of 439 amino acids with a molecular weight of 48 kDa. Expression of the gene in Escherichia coli resulted in the production of Tk0304 in soluble form which was purified by heat treatment at 80°C followed by ion exchange chromatography. Enzyme activity of Tk0304 was enhanced about 50% in the presence of 30 µM flavin adenine dinucleotide (FAD) when assay was conducted at 60°C. Surprisingly the activity of the enzyme was not affected by FAD when the assay was conducted at 75°C or at higher temperatures. Tk0304 displayed highest activity at pH 9 and 80°C. The enzyme was highly thermostable displaying 50% of the original activity even after an incubation of 80 min in boiling water. Among the potent inhibitors of NADH oxidases, silver nitrate and potassium cyanide did not show any significant inhibitory effect at a final concentration of 100 µM. -

DNA Polymerase I- Dependent Replication (Temperature-Sensitive Dna Mutants/Extragenic Suppression) OSAMI NIWA*, SHARON K
Proc. Nati Acad. Sci. USA Vol. 78, No. 11, pp. 7024-7027, November 1981 Genetics Alternate pathways of DNA replication: DNA polymerase I- dependent replication (temperature-sensitive dna mutants/extragenic suppression) OSAMI NIWA*, SHARON K. BRYAN, AND ROBB E. MOSES Department ofCell Biology, Baylor College of Medicine, Houston, Texas 77030 Communicated by D. Nathans, July 10, 1981 ABSTRACT We have previously shown that someEscherichia proceed in the presence of a functional DNA polymerase I ac- coli [derivatives of strain HS432 (polAl, polB100, polC1026)] can tivity, despite a ts DNA polymerase III (6). replicate DNA at a restrictive temperature in the presence of a We report here that DNA replication in the parent strain polCts mutation and that such revertants contain apparent DNA becomes temperature-resistant with introduction ofDNA poly- polymerase I activity. We demonstrate here that this strain ofE. merase I activity but is ts in the absence of DNA polymerase colibecomes temperature-resistant upon the introduction ofa nor- I or presence of a ts DNA polymerase I activity. We conclude mal gene for DNA polymerase I or suppression of the polAl non- that this strain contains a sense mutation. Such temperature-resistant phenocopies become mutation (pcbA-) that allows repli- temperature-sensitive upon introduction of a temperature-sensi- cation to be dependent on DNA polymerase I polymerizing tive DNA polymerase I gene. Our results confirm that DNA rep- activity. This locus can be transduced to other E. coli strains and lication is DNA polymerase I-dependent in the temperature-re- again exerts phenotypic suppression of the polCts mutation in sistant revertants, indicating that an alternative pathway of the presence of DNA polymerase I. -

Biotechnology DNA Polymerases
Paper No. : 04 Genetic engineering and recombinant DNA technology Module : 07 DNA polymerases Principal Investigator: Dr Vibha Dhawan, Distinguished Fellow and Sr. Director The Energy and Resouurces Institute (TERI), New Delhi Co-Principal Investigator: Prof S K Jain, Professor, of Medical Biochemistry Jamia Hamdard University, New Delhi Paper Coordinator: Dr Mohan Chandra Joshi, Assistant Professor, Jamia Millia Islamia, New Delhi Content Writer: Dr Samer Singh, Assistant Professor, Panjab University, Chandigarh Content Reviwer: Dr Mohan Chandra Joshi, Assistant Professor, Jamia Millia Islamia, New Delhi Genetic engineering and recombinant DNA technology Biotechnology DNA polymerases Description of Module Subject Name Biotechnology Paper Name Genetic engineering and recombinant DNA technology Module Name/Title DNA polymerases Module Id 07 Pre-requisites Basic DNA Structure and DNA replication Objectives 1) WHAT ARE DNA POLYMERASES? - DISCOVERY 2) TYPES in Escherichia coli 3) WHAT THEY DO? 4) STRUCTURE - CONSERVATION & FUNCTION 5) DNA POLYMERIZATION REACTION and ITS ATTRIBUTES 6) DNA POLYMERASE'S FIDELITY AND PROCESSIVITY 7) DNA POLYMERASE FAMILY AND EXAMPLES 8) HOW SPECIAL FUNCTION DNA POLYMERASE 'TELOMERASE' CARRIES OUT LENGTHENING OF LINEAR DNA ENDS Keywords DNA polymerase, reverse transcriptase, DNA polymerization, Processivity, Fidelity, Telomerase Genetic engineering and recombinant DNA technology Biotechnology DNA polymerases Table of contents A. Learning Objectives B. Keywords C. DNA POLYMERASES C1. General Overview -Discovery C2. Comparison of DNA polymerases of Escherichia coli C3. DNA POLYMERASE CORE STRUCTURE & FUNCTION C4. DNA POLYMERIZATION ACTIVITY and ITS ATTRIBUTES C5. DNA POLYMERASE FAMILIES C6. TELOMERASE- A special function DNA polymerase in eukaryotes D. Summary A. LEARNING OBJECTIVES In this module, Students will learn following: 1. WHAT ARE DNA POLYMERASES? - DISCOVERY 2. -

Pyrococcus Horikoshii Rada Intein Site Selection
Murray State's Digital Commons Honors College Theses Honors College Fall 11-12-2020 Pyrococcus horikoshii RadA Intein Site Selection Noah Stallins Follow this and additional works at: https://digitalcommons.murraystate.edu/honorstheses Part of the Biology Commons Recommended Citation Stallins, Noah, "Pyrococcus horikoshii RadA Intein Site Selection" (2020). Honors College Theses. 61. https://digitalcommons.murraystate.edu/honorstheses/61 This Thesis is brought to you for free and open access by the Honors College at Murray State's Digital Commons. It has been accepted for inclusion in Honors College Theses by an authorized administrator of Murray State's Digital Commons. For more information, please contact [email protected]. Murray State University Honors College HONORS THESIS Certificate of Approval Pyrococcus horikoshii RadA Intein Site Selection Noah Reed Stallins November 2020 Approved to fulfill the requirements of HON 437 __________________________________________ Professor Dr. Christopher Lennon, Assistant Professor Biology Approved to fulfill the Honors Thesis requirement __________________________________________ Of the Murray State Honors Dr. Warren Edminster, Executive Director College Honors College Examination Approval Page Author: Noah Reed Stallins Project Title: Pyrococcus horikoshii RadA Intein Site Selection Department: Biology Date of Defense: 11/12/2020 Approval by Examining Committee: _____________________________ ________ (Dr. Christopher Lennon, Advisor) (Date) ________________________________ ________ (Dr. Gary ZeRuth, Committee Member) (Date) _____________________________ ________ (Dr. Terry Derting, Committee Member) (Date) Pyrococcus horikoshii RadA Intein Site Selection Submitted in partial fulfillment Of the requirements Of the Murray State University Honors Diploma Noah Reed Stallins November 2020 ABSTRACT Inteins are protein segments interrupting polypeptides with the unique ability to excise from the host protein and link flanking protein fragments (exteins) to form a functional protein. -

Table 4. V. Cholerae Flexgene ORF Collection
Table 4. V. cholerae FLEXGene ORF collection Reference Clone protein PlasmID clone GenBank Locus tag Symbol accession identifier FLEX clone name accession Product name VC0001 NP_062585 VcCD00019918 FLH200476.01F DQ772770 hypothetical protein VC0002 mioC NP_062586 VcCD00019938 FLH200506.01F DQ772771 mioC protein VC0003 thdF NP_062587 VcCD00019958 FLH200531.01F DQ772772 thiophene and furan oxidation protein ThdF VC0004 yidC NP_062588 VcCD00019970 FLH200545.01F DQ772773 inner membrane protein, 60 kDa VC0005 NP_062589 VcCD00061243 FLH236482.01F DQ899316 conserved hypothetical protein VC0006 rnpA NP_062590 VcCD00025697 FLH214799.01F DQ772774 ribonuclease P protein component VC0007 rpmH NP_062591 VcCD00061229 FLH236450.01F DQ899317 ribosomal protein L34 VC0008 NP_062592 VcCD00019917 FLH200475.01F DQ772775 amino acid ABC transporter, ATP-binding protein VC0009 NP_062593 VcCD00019966 FLH200540.01F DQ772776 amino acid ABC transproter, permease protein VC0010 NP_062594 VcCD00019152 FLH199275.01F DQ772777 amino acid ABC transporter, periplasmic amino acid-binding portion VC0011 NP_062595 VcCD00019151 FLH199274.01F DQ772778 hypothetical protein VC0012 dnaA NP_062596 VcCD00017363 FLH174286.01F DQ772779 chromosomal DNA replication initiator DnaA VC0013 dnaN NP_062597 VcCD00017316 FLH174063.01F DQ772780 DNA polymerase III, beta chain VC0014 recF NP_062598 VcCD00019182 FLH199319.01F DQ772781 recF protein VC0015 gyrB NP_062599 VcCD00025458 FLH174642.01F DQ772782 DNA gyrase, subunit B VC0016 NP_229675 VcCD00019198 FLH199346.01F DQ772783 hypothetical protein