Chapter 9 Compressible Flow
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

O N Viscous, Inviscid and Centrifugal Instability M
On viscous, inviscid and centrifugal instability mechanisms in compressible boundary layers, including non-linear vortex/wave interaction and the effects of large Mach number on transition. Submitted to the University of London as a thesis for the degree of Doctor of Philosophy. Nicholas Derick Blackaby, University College. March 1991. 1 ProQuest Number: 10609861 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 10609861 Published by ProQuest LLC(2017). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 A b stract The stability and transition of a compressible boundary layer, on a flat or curved surface, is considered using rational asymptotic theories based on the large size of the Reynolds numbers of concern. The Mach number is also treated as a large parameter with regard to hypersonic flow. The resulting equations are simpler than, but consistent with, the full Navier Stokes equations, but numer ical computations are still required. This approach also has the advantage that particular possible mechanisms for instability and/or transition can be studied, in isolation or in combination, allowing understanding of the underlying physics responsible for the breakdown of a laminar boundary layer. -

Pub Date Abstract
DOCUMENT RESUME ED 058 456 VT 014 597 TITLE Exploring in Aeronautics. An Introductionto Aeronautical Sciences. INSTITUTION National Aeronautics and SpaceAdministration, Cleveland, Ohio. Lewis Research Center. REPORT NO NASA-EP-89 PUB DATE 71 NOTE 398p. AVAILABLE FROMSuperintendent of Documents, U.S. GovernmettPrinting Office, Washington, D.C. 20402 (Stock No.3300-0395; NAS1.19;89, $3.50) EDRS PRICE MF-$0.65 HC-$13.16 DESCRIPTORS Aerospace Industry; *AerospaceTechnology; *Curriculum Guides; Illustrations;*Industrial Education; Instructional Materials;*Occupational Guidance; *Textbooks IDENTIFIERS *Aeronatics ABSTRACT This curriculum guide is based on a year oflectures and projects of a contemporary special-interestExplorer program intended to provide career guidance and motivationfor promising students interested in aerospace engineering and scientific professions. The adult-oriented program avoidstechnicality and rigorous mathematics and stresses real life involvementthrough project activity and teamwork. Teachers in high schoolsand colleges will find this a useful curriculum resource, w!th manytopics in various disciplines which can supplement regular courses.Curriculum committees, textbook writers and hobbyists will also findit relevant. The materials may be used at college level forintroductory courses, or modified for use withvocationally oriented high school student groups. Seventeen chapters are supplementedwith photographs, charts, and line drawings. A related document isavailable as VT 014 596. (CD) SCOPE OF INTEREST NOTICE The ERIC Facility has assigned this document for processing to tri In our judgement, thisdocument is also of interest to the clearing- houses noted to the right. Index- ing should reflect their special points of view. 4.* EXPLORING IN AERONAUTICS an introduction to Aeronautical Sciencesdeveloped at the NASA Lewis Research Center, Cleveland, Ohio. -

Potential Equations and Pressure Coefficient For
International Journal of Theoretical & Applied Sciences, 9(1): 35-42(2017) ISSN No. (Print): 0975-1718 ISSN No. (Online): 2249-3247 Potential Equations and Pressure Coefficient for Compressible Flow: Comparison between Compressible and Incompressible Flow in Aerodynamics Menka Yadav * and Santosh Kumar Yadav ** *Research Scholar, J.J.T. University. Rajasthan, India ** Director (A&R), J.J.T. University, Rajasthan, India (Corresponding author: (Corresponding author: Menka Yadav) (Received 02 March, 2017 accepted 05 April, 2017) (Published by Research Trend, Website: www.researchtrend.net) ABSTRACT: We derive the potential equation for slender bodies and seek to understand the flow field equations for subsonic, supersonic and transonic flow in framework of small perturbation. Large amount of heat and mass can be transferred in a efficient way between the surface and fluid when flow is released against the surface. When aircraft passes through several distinct regions, the flow develops a velocity and pressure profile. In stagnation-region large scale turbulent flow affects transfer coefficient. At the face of object total pressure is higher than behind the object. Profile slopes shows that compressible and incompressible flows are related via certain equations. Zero Mach number incompressible medium causes pressure disturbances to move uniformly in all directions. Flow of heat and mass transfer is strongly affected by the geometry of the device. Keywords: Mach number, Pressure drag, Shock wave, Slender bodies, Velocity profile. I. INTRODUCTION called lift, which acts on the wing. Velocity varies along the wing chord and in the direction normal to its surface. Flow having significant changes in fluid density are The region adjacent to the wall, where the velocity known as compressible flow or flow with Mach number increases from zero to freestream value is known as the greater than 0.3 is treated as compressible. -

Eberhard Schneider, Klaus Thoma the ERNST-MACH-INSTITUT: PRESENT FIELDS of RESEARCH CONTINUATION of MACH’S SHOCK WAVE INVESTIGATIONS
Eberhard Schneider, Klaus Thoma THE ERNST-MACH-INSTITUT: PRESENT FIELDS OF RESEARCH CONTINUATION OF MACH’S SHOCK WAVE INVESTIGATIONS The present research program of the Ernst-Mach-Institut is presented. The Institute is one of 58 institutes of the Fraunhofer-Gesellschaft, all of which are working in fields of applied research. It was founded in 1959 by Hubert Schardin, a famous physicist working in fluid dynamics and ballistics. The institute’s name was chosen in honour of the great achievements and merits in physics of Ernst Mach, especially in the fields of shock waves in air and high speed photography. It is presented how these topics have been further investigated for many years. Shock wave studies have also been extended for solid materials by means of special acceleration techniques. The main tools for this purpose are a variety of light gas guns - their operation principle is explained - forming a unique research facility in Europe. Respective masses of milligrams up to many kilograms can be accelerated from m/s up to 10 km/s. The main applications for this technology are the following: Experimental space debris simulation for spacecraft protection; Simulation of natural high-speed impact phenomena; Ballistic missile defence tests; Terminal ballistic research. Other fields of work at the institute are the following: Dynamic material testing at very high deformation rates; Detonics; Development of high-speed visualization and testing equipment; Development of numerical simulation methods and material models; Security research. A fundamental working principle of the institute is doing both numerical and experimental studies in combination, whenever possible. SCHNEIDER, Eberhard a Klaus THOMA. -

Interaction Between a Conical Shock Wave and a Plane Compressible Turbulent Boundary Layer at Mach 2.05
INTERACTION BETWEEN A CONICAL SHOCK WAVE AND A PLANE COMPRESSIBLE TURBULENT BOUNDARY LAYER AT MACH 2.05 BY JASON T. HALE THESIS Submitted in partial fulfillment of the requirements for the degree of Master of Science in Aerospace Engineering in the Graduate College of the University of Illinois at Urbana-Champaign, 2014 Urbana, Illinois Advisers: Professor Gregory Elliott Professor J. Craig Dutton ABSTRACT The interaction between an impinging conical shock wave with a plane compressible turbulent boundary layer has been studied at Mach 2.05. Surface oil flow and pressure-sensitive paint (PSP) data were obtained beneath the oncoming boundary layer, while schlieren and particle image velocimetry (PIV) data were obtained in the streamwise/wall-normal (x-y) plane. Oil flow data suggested that the interaction causes two-dimensional (2D) separation near the centerline, and outside of this region three-dimensional (3D) separation that propagates fluid away from the centerline toward the sidewall. PSP results showed relatively constant upstream-influence length across the inviscid shock trace. PSP also revealed significant spanwise and streamwise expansion just downstream of the shock trace, unlike the qualitatively similar two-dimensional, wedge-generated oblique shock/boundary-layer interaction. Schlieren data suggested that the flow through the interaction was unseparated, and that there is significant unsteadiness in interaction position away from the centerline due to variation in the incoming boundary layer. PIV data showed the convection of large-scale vortical structures at velocities on the order of the streamwise velocity at the vortex center. These structures were smoothed out in the interaction. The PIV data moreover confirmed the downstream expansion shown by the PSP as well as a mean lack of flow separation. -

Lecture 1 COMPRESSIBLE FLOWS (Fundamental Aspects: Part - I)
NPTEL – Mechanical – Principle of Fluid Dynamics Module 4 : Lecture 1 COMPRESSIBLE FLOWS (Fundamental Aspects: Part - I) Overview In general, the liquids and gases are the states of a matter that comes under the same category as “fluids”. The incompressible flows are mainly deals with the cases of constant density. Also, when the variation of density in the flow domain is negligible, then the flow can be treated as incompressible. Invariably, it is true for liquids because the density of liquid decreases slightly with temperature and moderately with pressure over a broad range of operating conditions. Hence, the liquids are considered as incompressible. On the contrary, the compressible flows are routinely defined as “variable density flows”. Thus, it is applicable only for gases where they may be considered as incompressible/compressible, depending on the conditions of operation. During the flow of gases under certain conditions, the density changes are so small that the assumption of constant density can be made with reasonable accuracy and in few other cases the density changes of the gases are very much significant (e.g. high speed flows). Due to the dual nature of gases, they need special attention and the broad area of in the study of motion of compressible flows is dealt separately in the subject of “gas dynamics”. Many engineering tasks require the compressible flow applications typically in the design of a building/tower to withstand winds, high speed flow of air over cars/trains/airplanes etc. Thus, gas dynamics is the study of fluid flows where the compressibility and the temperature changes become important. -

Compressible Flow Related Terms
COMPRESSIBLE FLOW RELATED TERMS Based on Information in Wikipedia, the free encyclopedia Prepared by Patrick H. Oosthuizen Department of Mechanical and Materials Engineering Queen’s University Kingston, Ontario, Canada August, 2009 CONTENTS 1. Area Rule 2. Atmospheric Reentry 3. Ballistic Reentry 4. Blast Wave 5. Choked Flow 6. Compressible Flow 7. Compressibility 8. Compressibility Factor 9. Critical Mach Number 10. DeLaval Nozzle 11. Drag Divergence Mach Number 12. Hypersonic 13. Interferometer 14. Jet Engine 15. Mach Wave 16. Moving Shock Wave 17. Oblique Shock Wave 18. Prandtl-Meyer Function 19. Prandtl-Meyer Wave 20. Real Gas 21. Rocket Engine Nozzle 22. Schlieren Photography 23. Shadowgraph 24. Shock Tube 25. Shock Wave 26. Sonic Boom 27. Sound Barrier 28. Speed of Sound 29. Subsonic and Transonic Wind-tunnels 30. Supercritical Airfoil 31. Supersonic 32. Supersonic Transport Aircraft 33. Supersonic Wind-tunnel 34. Transonic 35. Wave Drag Area rule From Wikipedia, the free encyclopedia The Whitcomb area rule, also called the transonic area rule, is a design technique used to reduce an aircraft's drag at transonic and supersonic speeds, particularly between Mach 0.8 and 1.2. This is the operating speed range of the majority of commercial and military fixed-wing aircraft today. Description The Sears-Haack body shape At high-subsonic flight speeds, supersonic airflow can develop in areas where the flow accelerates around the aircraft body and wings. The speed at which this occurs varies from aircraft to aircraft, and is known as the critical Mach number. The resulting shock waves formed at these points of supersonic flow can bleed away a considerable amount of power, which is experienced by the aircraft as a sudden and very powerful form of drag, called wave drag. -

Theoretical Paschen's Law Model for Aerospace
THEORETICAL PASCHEN’S LAW MODEL FOR AEROSPACE VEHICLES: VALIDATION EXPERIMENT by JAYSEN MULLIGAN AROCHE B.S. University of Central Florida, 2016 A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in the Department of Mechanical and Aerospace Engineering in the College of Engineering and Computer Science at the University of Central Florida Orlando, Florida Spring Term 2018 © 2018 Jaysen Mulligan Aroche ii ABSTRACT Aerospace vehicles often experience triboelectric charging while traversing the atmosphere. Triboelectric charging occurs when a material come into frictional contact with a different material. Aerospace vehicles triboelectrically charge due to frictional contact with dust and ice crystals suspended in the atmosphere. Launch vehicles traversing ice clouds in low-pressure atmosphere are especially prone to electrostatic discharge events (i.e. sparks). These conditions are hazardous and affect the vehicle’s launch commit criteria. In 2010, engineers from an ARES- I rocket launch reported concerns with triboelectric charging over their self-destruct system antenna. This concern was addressed by putting the antenna through harsh conditions in a laboratory environment. The need for laboratory testing could have been avoided if there was a mathematical model to predict these events. These discharge events can typically be predicted by the Classical Paschen’s Law, which relates discharge voltage to pressure, material and distance between the charged and ground surfaces (i.e. electrodes). However, the Classical Paschen’s Law does not capture any aerodynamic considerations such as large bulk flow and compressibility effects. It became apparent that a new model would be needed to predict a discharge voltage with aerodynamic considerations. -

Li, Guoshuai (2020) Experimental Studies on Shock Wave Interactions with Flexible Surfaces and Development of Flow Diagnostic Tools
Li, Guoshuai (2020) Experimental studies on shock wave interactions with flexible surfaces and development of flow diagnostic tools. PhD thesis. https://theses.gla.ac.uk/81703/ Copyright and moral rights for this work are retained by the author A copy can be downloaded for personal non-commercial research or study, without prior permission or charge This work cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Enlighten: Theses https://theses.gla.ac.uk/ [email protected] EXPERIMENTAL STUDIES ON SHOCK WAVE INTERACTIONS WITH FLEXIBLE SURFACES AND DEVELOPMENT OF FLOW DIAGNOSTIC TOOLS Submitted in fulfilment of the requirements for the degree of Doctor of Philosophy Guoshuai Li School of Engineering College of Science and Engineering University of Glasgow February 2020 I. Author’s Declaration I declare that, except where explicit reference is made to the contribution of others, this dissertation is the result of my own work and has not been submitted for any other degree at the University of Glasgow or any other institution. Signature: __________ Printed name: Guoshuai Li iii II. Abstract Nowadays, light-weight composite materials have increasingly used for high-speed flight vehicles to improve their performance and efficiency. At supersonic speed, sonic fatigue, panel flutter, severe instabilities, and even catastrophic structural failure would occur due to the shock wave impingement on several flexible components of a given structural system either internally or externally. -
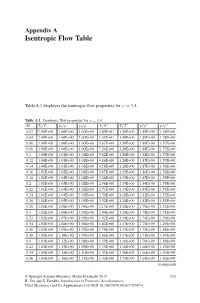
Appendix a Isentropic Flow Table
Appendix A Isentropic Flow Table TableA.1 displayes the isentropic flow properties for γ = 1.4. Table A.1 Isentropic flow properties for γ = 1.4 ∗ ∗ ∗ ∗ M Tt /T pt /p ρt /ρ A/A T/T p/p ρ/ρ 0.02 1.00E+00 1.00E+00 1.00E+00 2.89E+01 1.20E+00 1.89E+00 1.58E+00 0.04 1.00E+00 1.00E+00 1.00E+00 1.45E+01 1.20E+00 1.89E+00 1.58E+00 0.06 1.00E+00 1.00E+00 1.00E+00 9.67E+00 1.20E+00 1.89E+00 1.57E+00 0.08 1.00E+00 1.00E+00 1.00E+00 7.26E+00 1.20E+00 1.88E+00 1.57E+00 0.1 1.00E+00 1.01E+00 1.01E+00 5.82E+00 1.20E+00 1.88E+00 1.57E+00 0.12 1.00E+00 1.01E+00 1.01E+00 4.86E+00 1.20E+00 1.87E+00 1.57E+00 0.14 1.00E+00 1.01E+00 1.01E+00 4.18E+00 1.20E+00 1.87E+00 1.56E+00 0.16 1.01E+00 1.02E+00 1.01E+00 3.67E+00 1.19E+00 1.86E+00 1.56E+00 0.18 1.01E+00 1.02E+00 1.02E+00 3.28E+00 1.19E+00 1.85E+00 1.55E+00 0.2 1.01E+00 1.03E+00 1.02E+00 2.96E+00 1.19E+00 1.84E+00 1.55E+00 0.22 1.01E+00 1.03E+00 1.02E+00 2.71E+00 1.19E+00 1.83E+00 1.54E+00 0.24 1.01E+00 1.04E+00 1.03E+00 2.50E+00 1.19E+00 1.82E+00 1.53E+00 0.26 1.01E+00 1.05E+00 1.03E+00 2.32E+00 1.18E+00 1.81E+00 1.53E+00 0.28 1.02E+00 1.06E+00 1.04E+00 2.17E+00 1.18E+00 1.79E+00 1.52E+00 0.3 1.02E+00 1.06E+00 1.05E+00 2.04E+00 1.18E+00 1.78E+00 1.51E+00 0.32 1.02E+00 1.07E+00 1.05E+00 1.92E+00 1.18E+00 1.76E+00 1.50E+00 0.34 1.02E+00 1.08E+00 1.06E+00 1.82E+00 1.17E+00 1.75E+00 1.49E+00 0.36 1.03E+00 1.09E+00 1.07E+00 1.74E+00 1.17E+00 1.73E+00 1.48E+00 0.38 1.03E+00 1.10E+00 1.07E+00 1.66E+00 1.17E+00 1.71E+00 1.47E+00 0.4 1.03E+00 1.12E+00 1.08E+00 1.59E+00 1.16E+00 1.70E+00 1.46E+00 0.42 1.04E+00 1.13E+00 1.09E+00 1.53E+00 1.16E+00 1.68E+00 1.45E+00 0.44 1.04E+00 1.14E+00 1.10E+00 1.47E+00 1.16E+00 1.66E+00 1.43E+00 0.46 1.04E+00 1.16E+00 1.11E+00 1.42E+00 1.15E+00 1.64E+00 1.42E+00 (continued) © Springer Science+Business Media Dordrecht 2015 513 R. -

Compressible Flow
Compressible Flow Compressible FLow We know that fluids are classified as Incompressible and Compressible fluids. Incompressible fluids do not undergo significant changes in density as they flow. In general, liquids are incompressible;water being an excellent example. In contrast compressible fluids do undergo density changes. Gases are generally compressible;air being the most common compressible fluid we can find. Compressibility of gases leads to many interesting features such as shocks, which are absent for incompressible fluids. Gasdynamics is the discipline that studies the flow of compressible fluids and forms an important branch of Fluid Mechanics. In this book we give a broad introduction to the basics of compressible fluid flow. Figure 1.1: Classification of Fluids Though gases are compressible, the density changes they undergo at low speeds may not be considerable. Take air for instance. Fig. 1.2 shows the density changes plotted as a function of Mach Number. Density change is represented as where is the air density at zero speed (i.e., Zero Mach Number). file:///E|/Fluid%20Mechanics/Compressible%20Flow.htm (1 of 67)2/8/2007 1:12:29 AM Compressible Flow Figure 1.2: Density change as a function of Mach Number We observe that for Mach numbers up to 0.3, density changes are within about 5% of . So for all practical purposes one can ignore density changes in this region. But as the Mach Number increases beyond 0.3, changes do become appreciable and at a Mach Number of 1, it is 36.5% . it is interesting to note that at a Mach Number of 2, the density changes are as high as 77%. -

On Focusing of Shock Waves Veronica Eliasson
On focusing of shock waves by Veronica Eliasson September 2007 Technical Reports from Royal Institute of Technology KTH Mechanics SE-100 44 Stockholm, Sweden Akademisk avhandling som med tillst˚and av Kungliga Tekniska H¨ogskolan i Stockholm framl¨agges till offentlig granskning f¨or avl¨aggande av teknologie doktorsexamen fredagen den 21 september 2007 kl 10.15 i sal F3, Kungliga Tekniska H¨ogskolan, Valhallav¨agen 79, Stockholm. c Veronica Eliasson 2007 Universitetsservice US–AB, Stockholm 2007 On focusing of shock waves Veronica Eliasson KTH Mechanics, Royal Institute of Technology (KTH) SE-100 44 Stockholm, Sweden Abstract Both experimental and numerical investigations of converging shock waves have been performed. In the experiments, a shock tube was used to create and study converging shock waves of various geometrical shapes. Two methods were used to create polygonally shaped shocks. In the first method, the geometry of the outer boundary of the test section of the shock tube was varied. Four dif- ferent exchangeable shapes of the outer boundary were considered: a circle, a smooth pentagon, a heptagon, and an octagon. In the second method, an initially cylindrical shock wave was perturbed by metal cylinders placed in var- ious patterns and positions inside the test section. For three or more regularly spaced cylinders, the resulting diffracted shock fronts formed polygonal shaped patterns near the point of focus. Regular reflection was observed for the case with three cylinders and Mach refection was observed for cases with four or more cylinders. When the shock wave is close to the center of convergence, light emission is observed.