Morphometric Leaf Variation in Oaks (Quercus) of Bolu, Turkey
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Quercus Cerris
Quercus cerris Quercus cerris in Europe: distribution, habitat, usage and threats D. de Rigo, C. M. Enescu, T. Houston Durrant, G. Caudullo Turkey oak (Quercus cerris L.) is a deciduous tree native to southern Europe and Asia Minor, and a dominant species in the mixed forests of the Mediterranean basin. Turkey oak is a representative of section Cerris, a particular section within the genus Quercus which includes species for which the maturation of acorns occurs in the second year. Quercus cerris L., commonly known as Turkey oak, is a large fast-growing deciduous tree species growing to 40 m tall with 1 Frequency a trunk up to 1.5-2 m diameter , with a well-developed root < 25% system2. It can live for around 120-150 years3. The bark is 25% - 50% 50% - 75% mauve-grey and deeply furrowed with reddish-brown or orange > 75% bark fissures4, 5. Compared with other common oak species, e.g. Chorology Native sessile oak (Quercus petraea) and pedunculate oak (Quercus Introduced robur), the wood is inferior, and only useful for rough work such as shuttering or fuelwood1. The leaves are dark green above and grey-felted underneath6; they are variable in size and shape but are normally 9-12 cm long and 3-5 cm wide, with 7-9 pairs of triangular lobes6. The leaves turn yellow to gold in late autumn and drop off or persist in the crown until the next spring, especially on young trees3. The twigs are long and pubescent, grey or olive-green, with lenticels. The buds, which are concentrated Large shade tree in agricultural area near Altamura (Bari, South Italy). -

What Is a Tree in the Mediterranean Basin Hotspot? a Critical Analysis
Médail et al. Forest Ecosystems (2019) 6:17 https://doi.org/10.1186/s40663-019-0170-6 RESEARCH Open Access What is a tree in the Mediterranean Basin hotspot? A critical analysis Frédéric Médail1* , Anne-Christine Monnet1, Daniel Pavon1, Toni Nikolic2, Panayotis Dimopoulos3, Gianluigi Bacchetta4, Juan Arroyo5, Zoltán Barina6, Marwan Cheikh Albassatneh7, Gianniantonio Domina8, Bruno Fady9, Vlado Matevski10, Stephen Mifsud11 and Agathe Leriche1 Abstract Background: Tree species represent 20% of the vascular plant species worldwide and they play a crucial role in the global functioning of the biosphere. The Mediterranean Basin is one of the 36 world biodiversity hotspots, and it is estimated that forests covered 82% of the landscape before the first human impacts, thousands of years ago. However, the spatial distribution of the Mediterranean biodiversity is still imperfectly known, and a focus on tree species constitutes a key issue for understanding forest functioning and develop conservation strategies. Methods: We provide the first comprehensive checklist of all native tree taxa (species and subspecies) present in the Mediterranean-European region (from Portugal to Cyprus). We identified some cases of woody species difficult to categorize as trees that we further called “cryptic trees”. We collected the occurrences of tree taxa by “administrative regions”, i.e. country or large island, and by biogeographical provinces. We studied the species-area relationship, and evaluated the conservation issues for threatened taxa following IUCN criteria. Results: We identified 245 tree taxa that included 210 species and 35 subspecies, belonging to 33 families and 64 genera. It included 46 endemic tree taxa (30 species and 16 subspecies), mainly distributed within a single biogeographical unit. -

A Systems Biology Approach for Identifying Candidate Genes
A systems biology approach for identifying candidate genes involved in the natural variability of biomass yield and chemical properties in black poplar Vincent Segura, Marie-Claude Lesage Descauses, Jean-Paul Charpentier, Kévin Kinkel, Claire Mandin, Kévin Ader, Nassim Belmokhtar, Nathalie Boizot, Corinne Buret, Annabelle Dejardin, et al. To cite this version: Vincent Segura, Marie-Claude Lesage Descauses, Jean-Paul Charpentier, Kévin Kinkel, Claire Mandin, et al.. A systems biology approach for identifying candidate genes involved in the natural variability of biomass yield and chemical properties in black poplar. IUFRO Genomics and Forest Tree Genetics, May 2016, Arcachon, France. 2016, IUFRO Genomics and Forest Tree Genetics. Book of Abstract. hal-01456004 HAL Id: hal-01456004 https://hal.archives-ouvertes.fr/hal-01456004 Submitted on 3 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Abstract Book 1 Table of Contents Welcome……………………………………………………………………………………3 Presentation Abstracts……………………………………………………………………4 Opening Keynote Lecture………………………………………………………………...4 -

Notes Oak News
THE NEWSLETTER OF THE INTERNATIONAL OAK SOCIETY&, VOLUME 16, NO. 1, WINTER 2012 Greek OakOak Open Days: News September 26 - October Notes 2, 2011 From the 21st century CE to the 2nd century—BCE! The next morning early we met our large tour bus and its charming and skillful driver, Grigoris, who hails from the mountain village of Gardiki not far from here. We did a bit of leisurely botanizing before we reached Perdika, our first destination of the day. There are two reasons to visit Perdika: one is the Karavostasi beach, a curving strand with golden sand, and the archaeological site of Dymokastron, a Hellenis- tic mountain-top town reached by a steep hike. The view of the beach far below was beautiful, as it must have been when the town was still inhabited. The town was destroyed in 167 BCE by a Roman army, along with most of the other towns in the vicinity, all allied with Rome’s enemy, Macedonia. The site is under active excavation, and we were able to admire the remnants of protective walls (how in the world did they get those big stones up there?), building foundations, and cisterns, which were certainly needed in case of a prolonged siege, Some members of the IOS Greek tour relaxing under the plane tree in the which Dymocastron must have experienced more than once. village square. Vitsa, Epirus, Greece. (Photo: Gert Dessoy) The site also has many living trees, including wild pears (Py- rus spinosa Vill., also known as P. amygdaliformis Vill.) and uring this early autumn week of incomparable weather, figs (Ficus carica L.) which appear to be descendants of wild Dtwelve members of the IOS, and three others who were native trees selected by the original inhabitants, as well as guests, enjoyed a truly memorable time in northern Greece. -

New York City Approved Street Trees
New York City Approved Street Trees Suggested Tree Species Shape Visual interest Frequency of Preferred Cultivars Notes Scientific Name Common Name Planting Acer rubrum Red Maple Sparingly 'Red Sunset' ALB Host Aesculus hippocastanum Horsechestnut White May flowers Sparingly 'Baumanni' ALB Host Aesculus octandra Yellow Buckeye Yellow May Flowers Sparingly ALB Host ALB Host 'Duraheat' Betula nigra River Birch Ornamental Bark Sparingly Plant Single Stem 'Heritage' Only Celtis occidentalis Hackberry Ornamental Bark Sparingly 'Magnifica' ALB Host ALB Host Cercidiphyllum japonicum Katsura Tree Sparingly Plant Single Stem Only Corylus colurna Turkish Filbert Sparingly LARGE TREES: Mature LARGE TREES: height than greater feet 50 tall Eucommia ulmoides Hardy Rubber Tree Frequently 'Asplenifolia' Fagus sylvatica European Beech Sparingly 'Dawyckii Purple' 'Autumn Gold' Ginkgo biloba Ginkgo Yellow Fall Color Moderately 'Magyar' Very Tough Tree 'Princeton Sentry' 'Shademaster' 'Halka' Gleditsia triacanthos var inermis Honeylocust Yellow Fall Color Moderately 'Imperial' 'Skyline' 'Espresso' Gymnocladus dioicus Kentucky Coffeetree Large Tropical Leaves Frequently 'Prairie Titan' Page 1 of 7 New York City Approved Street Trees Suggested Tree Species Shape Visual interest Frequency of Preferred Cultivars Notes Scientific Name Common Name Planting 'Rotundiloba' Seedless Cultivars Liquidambar styraciflua Sweetgum Excellent Fall Color Frequently 'Worplesdon' Preffered 'Cherokee' Orange/Green June Liriodendron tulipifera Tulip Tree Moderately Flowers Metasequoia -

From Genes to Genomes: Botanic Gardens Embracing New Tools for Conservation and Research Volume 18 • Number 1
Journal of Botanic Gardens Conservation International Volume 18 • Number 1 • February 2021 From genes to genomes: botanic gardens embracing new tools for conservation and research Volume 18 • Number 1 IN THIS ISSUE... EDITORS Suzanne Sharrock EDITORIAL: Director of Global Programmes FROM GENES TO GENOMES: BOTANIC GARDENS EMBRACING NEW TOOLS FOR CONSERVATION AND RESEARCH .... 03 Morgan Gostel Research Botanist, FEATURES Fort Worth Botanic Garden Botanical Research Institute of Texas and Director, GGI-Gardens NEWS FROM BGCI .... 06 Jean Linksy FEATURED GARDEN: THE NORTHWESTERN UNIVERSITY Magnolia Consortium Coordinator, ECOLOGICAL PARK & BOTANIC GARDENS .... 09 Atlanta Botanical Garden PLANT HUNTING TALES: GARDENS AND THEIR LESSONS: THE JOURNAL OF A BOTANY STUDENT Farahnoz Khojayori .... 13 Cover Photo: Young and aspiring scientists assist career scientists in sampling plants at the U.S. Botanic Garden for TALKING PLANTS: JONATHAN CODDINGTON, the Global Genome Initiative (U.S. Botanic Garden). DIRECTOR OF THE GLOBAL GENOME INITIATIVE .... 16 Design: Seascape www.seascapedesign.co.uk BGjournal is published by Botanic Gardens Conservation International (BGCI). It is published twice a year. Membership is open to all interested individuals, institutions and organisations that support the aims of BGCI. Further details available from: ARTICLES • Botanic Gardens Conservation International, Descanso House, 199 Kew Road, Richmond, Surrey TW9 3BW UK. Tel: +44 (0)20 8332 5953, Fax: +44 (0)20 8332 5956, E-mail: [email protected], www.bgci.org BANKING BOTANICAL BIODIVERSITY WITH THE GLOBAL GENOME • BGCI (US) Inc, The Huntington Library, BIODIVERSITY NETWORK (GGBN) Art Collections and Botanical Gardens, Ole Seberg, Gabi Dröge, Jonathan Coddington and Katharine Barker .... 19 1151 Oxford Rd, San Marino, CA 91108, USA. -
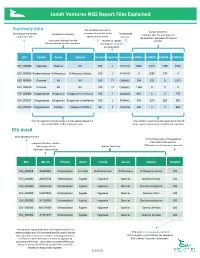
NGS Report Explained
Jonah Ventures NGS Report Files Explained Summary data The % of base pairs in the Sample identifiers Exact Sequence Variant sequence that match to the The detected Exact Sequence Variant Consensus Taxonomy The detected Numbers after the decimal point unique identifier species in the library. sequence. represent lab replicates of the same Taxonomic ranking from the Number of species sample. library matched to each sequence. matching the sequence at a given level. ESV Family Genus Species %match # Species Sequence S10001.1 S10002.1 S10003.1 S10003.2 ESV_000080 Fagaceae Quercus NA 100 7 AAAAAG… 3066 1340 5780 3462 ESV_000818 Orobanchaceae Orthocarpus Orthocarpus luteus 100 1 AAAAAG… 0 2582 249 0 ESV_000006 Poaceae NA NA 100 77 GAAAAG… 584 101 0 1039 ESV_000050 Poaceae NA NA 100 12 GAAAAG… 1328 0 0 0 ESV_000308 Polygonaceae Polygonum Polygonum humifusum 100 2 AAAAAG… 902 0 0 278 ESV_000027 Polygonaceae Eriogonum Eriogonum umbellatum 100 1 ATAAAG… 341 124 282 381 ESV_000520 Polygonaceae Fallopia Fallopia multiflora 99 1 AAAAAG… 126 0 0 994 “NA” at a taxonomic ranks means multiple species present in The number in each cell is the absolute number of the sample differ in that taxonomic rank times a given sequence was read by the sequencer. ESV detail Exact Sequence Variant The % of base pairs in the sequence that match to the species. Index identification number. that match to the species. Each sample has an Species Taxonomy 100% indicates all base pairs matched individual index number. ESV GB_ID Phylum Order Family Genus Species %match ESV_000818 -

(Quercus Cerris, Q. Frainetto, Q. Robur) and the Optimization Perspective of Growth Conditions for in Vitro Propagated Plants Transplanted in the Field
Ann. For. Res. 54(1): 57-71, 2011 ANNALS OF FOREST RESEARCH www.e-afr.org Mycorrhizal status of several Quercus species in Romania (Quercus cerris, Q. frainetto, Q. robur) and the optimization perspective of growth conditions for in vitro propagated plants transplanted in the field E. Fodor, A. Timofte, T. Geambau Fodor E., Timofte A., Geambau T. 2011. Mycorrhizal status of several Quercus species in Romania (Quercus cerris, Q. frainetto, Q. robur) and the optimization perspective of growth conditions for in vitro propagated plants transplanted in the [ eld. Ann. For. Res. 54(1): 57-71, 2011. Abstract. There is an increasing interest for important tree species conserva- tion in the context of climate change, anthropogenic pressure and invasion of alien tree species. A key factor in the survival of trees is represented by the mycorrhizal association. The success of micropropagated trees also de- pends on the acquisition of mycorrhizal mutualists. Ectomycorrhizal roots samples from several Quercus species (Q. cerris, Q. frainetto, Q. robur) were examined for mycorrhizal morphotypes’ characterization. The sam- ples were collected during the vegetation season from stands located in Southern and North-Western Romania. 30 morphotypes of active mycor- rhizae were identified with Cenococcum geophilum Fr. (Ascomycota) as dominating morphotype. Previous studies on somatic embryogenesis in Q. robur and Q. frainetto demonstrated the utility of in vitro techniques in obtaining plants from these recalcitrant seed producing species, con- sidered at risk in various areas of the country, due to increasingly stress- ful conditions. The success rate of the acclimatization process depends on the mycorrhization performed either artificially, in the laboratory, ei- ther naturally, in the field. -

Response of Downy Oak (Quercus Pubescens Willd.) to Climate Change: Transcriptome Assembly, Differential Gene Analysis and Targe
plants Article Response of Downy Oak (Quercus pubescens Willd.) to Climate Change: Transcriptome Assembly, Differential Gene Analysis and Targeted Metabolomics Jean-Philippe Mevy 1,*, Beatrice Loriod 2, Xi Liu 3, Erwan Corre 3, Magali Torres 2, Michael Büttner 4, Anne Haguenauer 1, Ilja Marco Reiter 5, Catherine Fernandez 1 and Thierry Gauquelin 1 1 CNRS, Aix-Marseille University, Avignon University, IRD, IMBE, 13331 Marseille, France; [email protected] (A.H.); [email protected] (C.F.); [email protected] (T.G.) 2 TGML-TAGC—Inserm UMR1090 Aix-Marseille Université 163 avenue de Luminy, 13288 Marseille, France; [email protected] (B.L.); [email protected] (M.T.) 3 CNRS, Sorbonne Université, FR2424, ABiMS platform, Station Biologique, 29680 Roscoff, France; xi.liu@sb-roscoff.fr (X.L.); erwan.corre@sb-roscoff.fr (E.C.) 4 Metabolomics Core Technology Platform Ruprecht-Karls-University Heidelberg Centre for Organismal Studies (COS) Im Neuenheimer Feld 360, 69120 Heidelberg, Germany; [email protected] 5 Research Federation ECCOREV FR3098, CNRS, 13545 Aix-en-Provence, France; [email protected] * Correspondence: [email protected]; Tel.: +33-0413550766 Received: 20 July 2020; Accepted: 1 September 2020; Published: 4 September 2020 Abstract: Global change scenarios in the Mediterranean basin predict a precipitation reduction within the coming hundred years. Therefore, increased drought will affect forests both in terms of adaptive ecology and ecosystemic services. However, how vegetation might adapt to drought is poorly understood. In this report, four years of climate change was simulated by excluding 35% of precipitation above a downy oak forest. -

Redalyc.Tree and Tree-Like Species of Mexico: Apocynaceae, Cactaceae
Revista Mexicana de Biodiversidad ISSN: 1870-3453 [email protected] Universidad Nacional Autónoma de México México Ricker, Martin; Valencia-Avalos, Susana; Hernández, Héctor M.; Gómez-Hinostrosa, Carlos; Martínez-Salas, Esteban M.; Alvarado-Cárdenas, Leonardo O.; Wallnöfer, Bruno; Ramos, Clara H.; Mendoza, Pilar E. Tree and tree-like species of Mexico: Apocynaceae, Cactaceae, Ebenaceae, Fagaceae, and Sapotaceae Revista Mexicana de Biodiversidad, vol. 87, núm. 4, diciembre, 2016, pp. 1189-1202 Universidad Nacional Autónoma de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=42548632003 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Available online at www.sciencedirect.com Revista Mexicana de Biodiversidad Revista Mexicana de Biodiversidad 87 (2016) 1189–1202 www.ib.unam.mx/revista/ Taxonomy and systematics Tree and tree-like species of Mexico: Apocynaceae, Cactaceae, Ebenaceae, Fagaceae, and Sapotaceae Especies arbóreas y arborescentes de México: Apocynaceae, Cactaceae, Ebenaceae, Fagaceae y Sapotaceae a,∗ b a a Martin Ricker , Susana Valencia-Avalos , Héctor M. Hernández , Carlos Gómez-Hinostrosa , a b c Esteban M. Martínez-Salas , Leonardo O. Alvarado-Cárdenas , Bruno Wallnöfer , a a Clara H. Ramos , Pilar E. Mendoza a Herbario Nacional de México (MEXU), Departamento -

And Natural Community Restoration
RECOMMENDATIONS FOR LANDSCAPING AND NATURAL COMMUNITY RESTORATION Natural Heritage Conservation Program Wisconsin Department of Natural Resources P.O. Box 7921, Madison, WI 53707 August 2016, PUB-NH-936 Visit us online at dnr.wi.gov search “ER” Table of Contents Title ..……………………………………………………….……......………..… 1 Southern Forests on Dry Soils ...................................................... 22 - 24 Table of Contents ...……………………………………….….....………...….. 2 Core Species .............................................................................. 22 Background and How to Use the Plant Lists ………….……..………….….. 3 Satellite Species ......................................................................... 23 Plant List and Natural Community Descriptions .…………...…………….... 4 Shrub and Additional Satellite Species ....................................... 24 Glossary ..................................................................................................... 5 Tree Species ............................................................................... 24 Key to Symbols, Soil Texture and Moisture Figures .................................. 6 Northern Forests on Rich Soils ..................................................... 25 - 27 Prairies on Rich Soils ………………………………….…..….……....... 7 - 9 Core Species .............................................................................. 25 Core Species ...……………………………….…..…….………........ 7 Satellite Species ......................................................................... 26 Satellite Species -

Appendix G – Right-Of-Way Street Tree List
AS ADOPTED BY THE PARKS, RECREATION AND CULTURAL SERVICES BOARD ON DECEMBER 6, 2018 APPENDIX G – RIGHT-OF-WAY STREET TREE LIST APPENDIX G – RIGHT-OF-WAY STREET TREE LIST 1 Large Columnar Trees Scientific & Common Mature Spread Under Min Strip Flower Fall Comments Name Height (ft) Wires/View Width (ft) Color Color (ft) Covenants Acer nigrum ‘Green 50 10 No 6 N/A Good close to Column’ buildings Green Column Black Sugar Maple Ginko biloba ‘Princeton 40 15 No 6 N/A Very narrow growth. Sentry’ Princeton Sentry Ginkgo Nyssa sylvatica 60 20 No 6 N/A Handsome chunky Tupelo bark – Great Plant Pick Quercus ‘Crimschmidt’ 45 15 No 6 N/A Hard to find in the Crimson Spire Oak nursery trade Quercus frainetto 50 30 No 6 N/A Drought resistant – Italian Oak beautiful green, glossy leaves in summer. Great Plant Pick Quercus robur 40 15 No 6 N/A Columnar variety of ‘fastigiata’ oak Skyrocket Oak Taxodium distichum 55 20 No 6 N/A Deciduous conifer - 'Mickelson' Shawnee tolerates city Brave Bald Cypress conditions Large Trees Scientific & Common Mature Spread Under Min Flower Fall Comments Name Height (ft) Wires/View Strip Color Color (ft) Covenants Width (ft) Acer saccharum Fastest growing sugar ‘Bonfire’ 50 40 No 6 N/A maple Bonfire Sugar Maple Acer saccharum Resistant to leaf tatter. 'Commemoration' Great Plant Pick 50 35 No 6 N/A Commemoration Sugar Maple Acer saccharum 'Green Reliable fall color. Mountain' Green 45 35 No 6 N/A Great Plant Pick Mountain Sugar Maple Acer saccharum Limited use - where 'Legacy' sugar maple is desired 50 35 No 5 N/A Legacy Sugar Maple in limited planting strip area.