AOAC Stakeholder Panel on Dietary Supplements Stakeholder Panel Meeting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Dietary Supplements Compendium 2019 Edition
Products and Services New Dietary Supplements Reference Standards Below is a list of newly released Reference Standards. Herbal Medicines/ Botanical Dietary Supplements Baicalein Baicalein 7-O-Glucuronide Chebulagic Acid Dietary Supplements Compendium 2019 Edition Coptis chinensis Rhizome Dry Extract In response to the customer feedback, coupled with evolving information needs, USP moved the Psoralen Dietary Supplements Compendium (DSC) to an online platform for the 2019 edition. DSC continues Scutellaria baicalensis Root Dry to provide in-depth, comprehensive information for all phases of development and manufacturing of Extract quality dietary supplements including quality control, quality assurance, and regulatory/compendial Terminalia chebula Fruit Dry affairs. Extract Guarana Seed Dry Extract Some of the advantages that come with the new online DSC edition include: Cullen Corylifolium Fruit Dry Extract More frequent updates to ensure access to the most current information Procyanidin B2 Customizable alerts to notify of changes to selected documents An intuitive interface to facilitate quick and easy navigation Non-Botanicals A customizable workspace with bookmarks, alerts and a viewing history beta-Glycerylphosphorylcholine Convenient, anytime, anywhere access with common browsers Conjugated Linoleic Acids – In addition to selected new and revised monographs and General Chapters from the USP-NF and Triglycerides Food Chemicals Codex issued since the previous 2015 edition, the DSC 2019 features: Creatine Docosahexaenoic Acid 24 new General Chapters Eicosapentaenoic Acid 72 new dietary ingredient and dietary supplement monographs L-alpha- 27 sets of supplementary information for botanical and nonbotanical dietary supplements Glycerophosphorylethanolamine 59 updated botanical HPTLC plates L-alpha- Revised and updated dietary intake comparison tables Glycerylphosphorylcholine Updated Dietary Supplement Verification Program manual Omega-3 Free Fatty Acids Pyrroloquinoline Quinone View this page for more information or to subscribe to the 2019 online DSC. -

ADVISORY BOARDS Each Issue of Herbaigram Is Peer Reviewed by Various Members of Our Advisory Boards Prior to Publication
ADVISORY BOARDS Each issue of HerbaiGram is peer reviewed by various members of our Advisory Boards prior to publication . American Botanical Council Herb Research Dennis V. C. Awang, Ph.D., F.C.I.C., MediPiont Natural Gail B. Mahadr, Ph.D., Research Assistant Professor, Products Consul~ng Services, Ottowa, Ontario, Conodo Deportment o Medical Chemistry &Pharmacognosy, College of Foundation Pharmacy, University of Illinois, Chicago, Illinois Manuel F. Balandrin, R.Ph., Ph.D., Research Scien~st, NPS Rob McCaleb, President Pharmaceuticals, Salt LakeCity , Utah Robin J. Maries, Ph.D., Associate Professor of Botany, Brandon University, Brandon, Manitoba, Conodo Mi(hael J. Balidt, Ph.D., Director of the lns~tute of Econom ic Glenn Appelt, Ph.D., R.Ph., Author and Profess or Botany, the New York Botanical Gorden, Bronx, New York Dennis J. M(Kenna, Ph.D., Consulting Ethnophormocologist, Emeritus, University of Colorado, and with Boulder Beach Joseph M. Betz, Ph.D., Research Chemist, Center for Food Minneapolis, Minnesota Consulting Group Safety and Applied Nutri~on, Division of Natural Products, Food Daniel E. Moerman, Ph.D., William E. Stirton Professor of John A. Beutler, Ph.D., Natural Products Chemist, and Drug Administro~on , Washington, D.C. Anthropology, University of Michigon/ Deorbom, Dearborn, Michigan Notional Cancer Institute Donald J. Brown, N.D., Director, Natural Products Research Consultants; Faculty, Bastyr University, Seattle, Washington Samuel W. Page, Ph.D., Director, Division of Natural Products, Robert A. Bye, Jr., Ph.D., Professor of Ethnobotony, Notional University of Mexico Thomas J. Carlson, M.S., M.D., Senior Director, Center for Food Safety and Applied Nutri~on , Food and Drug Administro~on , Washington, D.C. -

Echinacea Purpurea Extracts (Echinacin) and Thymostimulin
SUMMARY OF DATA FOR CHEMICAL SELECTION Echinacea BASIS OF NOMINATION TO THE CSWG Echinacea is presented to the CSWG as part of a review of botanicals being used as dietary supplements in the United States. Alternative herbal medicines are projected to be a $5 billion market by the turn of the century. Echinacea is an extremely popular herbal supplement; sales are nearly $300 million a year according to the last figures available. Sweeping deregulation of botanicals now permits echinacea to be sold to the public without proof of safety or efficacy if the merchandiser notes on the label that the product is not intended to diagnose, treat, cure, or prevent any disease. The literature on echinacea clearly showed that it is being used for the treatment of viral and bacterial infections although virtually no information on safety was found. INPUT FROM GOVERNMENT AGENCIESIINDUSTRY According to the Center for Food Safety and Applied Nutrition, FDA does not have information about the safety or purported benefits of echinacea. SELECTION STATUS • ACTION BY CSWG: 4/28/98 Studies requested: - Toxicological evaluation, to include 90-day subchronic study - Carcinogenicity, depending on the results of the toxicologic evaluation - Micronucleus assay Priority: Moderate RationalelRemarks: - Potential for widespread human exposure. - Most popular herbal supplement in the US, used to stimulate immune system - Test material should be standardized to 2.4% P-l,2-d-fructofuranoside - NCI will conduct Ames Salmonella assay Echinacea CHEMICAL IDENTIFICATION Chemical Abstract Service Names: CAS Registry Numbers: Echinacea angustifolia, ext. 84696-11-7 Echinacea purpurea, ext. 90028-20-9 Echinacea pallida, ext. 97281-15-7 Echinacea angustifolia, tincture 129677-89-0 Description: Echinacea are herbaceous perennials of the daisy family. -
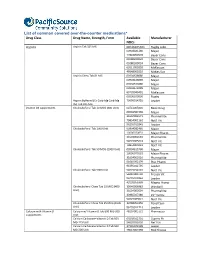
List of Common Covered Over-The-Counter Medications*
List of common covered over-the-counter medications* Drug Class Drug Name, Strength, Form Available Manufacturer NDCs Aspirin Aspirin Tab 325 MG 00536105301 Rugby Labo 00904681180 Major 12843054578 Bayer Cons 00280200020 Bayer Cons 00280200024 Bayer Cons 62011002003 McKesson 49348000110 McKes Sun Aspirin Chew Tab 81 MG 00904628880 Major 00904628889 Major 00904679480 Major 00904679489 Major 63739043401 McKesson 00536100836 Rugby Aspirin Buffered (Ca Carb-Mg Carb-Mg 70000014701 Leader Ox) Tab 325 MG Vitamin D3 supplements Cholecalciferol Tab 10 MCG (400 Unit) 00761005820 Basic Drug 00904582360 Major 31604002671 PharmaVite 79854001162 Nat’l Vit 96295012845 Leader Cholecalciferol Tab 1000 Unit 00904582460 Major 10006070033 Major Pharm 31604002683 PharmaVite 54629005024 Nat’l Vit 79854005023 Nat’l Vit Cholecalciferol Tab 50 MCG (2000 Unit) 00904615760 Major 10006070162 Major Pharm 31604002516 PharmaVite 51645092199 Plus Pharm 96295012795 Leader Cholecalciferol Tab 5000 Unit 54629794101 Nat’l Vit 58487003702 Freeda Vit 96295012846 Leader 43292056338 Magno-Hump Cholecalciferol Chew Tab 10 MCG (400 35046006982 Windwill Unit) 31604002604 PharmaVite 40985027380 21st Centu 54629089821 Nat’l Vit Cholecalciferol Chew Tab 25 MCG (1000 30768050356 Rexall Sun Unit) 96295012713 Leader Calcium with Vitamin D Calcium w/ Vitamin D Tab 600 MG-200 48107005122 Pharmassur supplements Unit Calcium Carbonate-Vitamin D Tab 500 60258012701 Cypress Ph MG-125 Unit 54629031630 Nat’l Vit Calcium Carbonate-Vitamin D Tab 500 37205043198 Leader MG-200 Unit 49614067298 -

Petition for a Writ of Mandamus
Case: 18-114 Document: 2-1 Page: 1 Filed: 12/01/2017 (1 of 257) No. 17-______ IN THE UNITED STATES COURT OF APPEALS FOR THE FEDERAL CIRCUIT ________________ IN RE AMARIN PHARMA, INC. AND AMARIN PHARMACEUTICALS IRELAND LTD., Petitioners. ________________ On Petition for a Writ of Mandamus to the United States International Trade Commission, In the Matter of Certain Synthetically Produced, Predominantly EPA Omega-3 Products in Ethyl Ester or Re-esterified Triglyceride Form, ITC Docket No. 3247. ________________ PETITION FOR A WRIT OF MANDAMUS ________________ Of Counsel: Ashley C. Parrish Joseph T. Kennedy Principal Counsel Barbara Kurys Jeffrey M. Telep AMARIN PHARMA, INC. Lisa M. Dwyer* 1430 Route 206 Jesse Snyder Bedminster, NJ 07921 KING & SPALDING LLP Telephone: (908) 719-1315 1700 Pennsylvania Ave. N.W. Facsimile: (908) 719-3012 Washington, DC 20006-4706 Telephone: (202) 737-0500 Facsimile: (202) 626-3737 Email: [email protected] Counsel for Petitioners Amarin Pharma, Inc. and Amarin Pharmaceuticals Ireland Ltd. December 1, 2017 *Admission Pending Case: 18-114 Document: 2-1 Page: 2 Filed: 12/01/2017 (2 of 257) CERTIFICATE OF INTEREST Counsel for Amarin Pharma, Inc. and Amarin Pharmaceuticals Ireland Ltd. certify the following: 1. The full name of every party or amicus represented herein: Amarin Pharma, Inc. and Amarin Pharmaceuticals Ireland Ltd. 2. The name of the real party in interest (if the party named in the caption is not the real party in interest) represented us: Not applicable. 3. All parent corporations and any publicly held companies that own 10 percent or more of the stock of the party or amicus curiae represented herein: Amarin Pharma, Inc. -

An Analysis of the Pollinators of Echinacea Purpurea in Relation to Their Perceived Efficiency and Color Preferences
University of Tennessee at Chattanooga UTC Scholar Student Research, Creative Works, and Honors Theses Publications 5-2021 An analysis of the pollinators of Echinacea purpurea in relation to their perceived efficiency and color efpr erences Carmen Black University of Tennessee at Chattanooga, [email protected] Follow this and additional works at: https://scholar.utc.edu/honors-theses Part of the Botany Commons Recommended Citation Black, Carmen, "An analysis of the pollinators of Echinacea purpurea in relation to their perceived efficiency and color efpr erences" (2021). Honors Theses. This Theses is brought to you for free and open access by the Student Research, Creative Works, and Publications at UTC Scholar. It has been accepted for inclusion in Honors Theses by an authorized administrator of UTC Scholar. For more information, please contact [email protected]. An Analysis of the Pollinators of Echinacea purpurea in Relation to their Perceived Efficiency and Color Preferences Departmental Honors Thesis The University of Tennessee at Chattanooga Department of Biology, Geology, and Environmental Sciences Examination Date: April 6th Dr. Stylianos Chatzimanolis Dr. Joey Shaw Professor of Biology Professor of Biology Thesis Director Department Examiner Dr. Elise Chapman Lecturer of Biology Department Examiner 2 TABLE OF CONTENTS I. Abstract …………..…………………….………………………… 3 II. Introduction…………..………………….……………………....... 5 III. Materials and Methods…………...………………………………. 11 IV. Results…………..…………………….………………………….. 16 A. List of Figures…………...……………………………….. 21 V. Discussion…………..………….…………………………...…… 28 VI. Acknowledgements………….……………….………...………… 38 VII. Works Cited ……………………………………...……….……… 39 VIII. Appendices……………………………………………………….. 43 3 ABSTRACT This study aimed to better understand how insects interacted with species of Echinacea in Tennessee and specifically their preference to floral color. Based on previous studies I expected the main visitors to be composed of various bees, beetles and butterflies. -

Value Addition of Southern African Monkey Orange (Strychnos Spp.): Composition, Utilization and Quality Ruth Tambudzai Ngadze
Value addition of Southern African monkey orange ( Value addition of Southern African monkey orange (Strychnos spp.): composition, utilization and quality Strychnos spp.): composition, utilization and quality Ruth Tambudzai Ngadze 2018 Ruth Tambudzai Ngadze Propositions 1. Food nutrition security can be improved by making use of indigenous fruits that are presently wasted, such as monkey orange. (this thesis) 2. Bioaccessibility of micronutrients in maize-based staple foods increases by complementation with Strychnos cocculoides. (this thesis) 3. The conclusion from Baker and Oswald (2010) that social media improve connections, neglects the fact that it concomitantly promotes solitude. (Journal of Social and Personal Relationships 27:7, 873–889) 4. Sustainable agriculture in developed countries can be achieved by mimicking third world small-holder agrarian systems. 5. Like first time parenting, there is no real set of instructions to prepare for the PhD journey. 6. Undertaking a sandwich PhD is like participating in a survival reality show. Propositions belonging to the thesis, entitled: Value addition of Southern African monkey orange (Strychnos spp.): composition, utilization and quality Ruth T. Ngadze Wageningen, October 10, 2018 Value addition of Southern African monkey orange (Strychnos spp.): composition, utilization and quality Ruth Tambudzai Ngadze i Thesis committee Promotor Prof. Dr V. Fogliano Professor of Food Quality and Design Wageningen University & Research Co-promotors Dr A. R. Linnemann Assistant professor, Food Quality and Design Wageningen University & Research Dr R. Verkerk Associate professor, Food Quality and Design Wageningen University & Research Other members Prof. M. Arlorio, Università degli Studi del Piemonte Orientale A. Avogadro, Italy Dr A. Melse-Boonstra, Wageningen University & Research Prof. -

Isolation and Functional Characterization of a Cdna Coding A
Isolation and functional characterization of a cDNA coding a hydroxycinnamoyltransferase involved in phenylpropanoid biosynthesis in Cynara cardunculus L Cinzia Comino, Sergio Lanteri, Ezio Portis, Alberto Acquadro, Annalisa Romani, Alain Hehn, Romain Larbat, Frédéric Bourgaud To cite this version: Cinzia Comino, Sergio Lanteri, Ezio Portis, Alberto Acquadro, Annalisa Romani, et al.. Isolation and functional characterization of a cDNA coding a hydroxycinnamoyltransferase involved in phenyl- propanoid biosynthesis in Cynara cardunculus L. BMC Plant Biology, BioMed Central, 2007, 7 (1), pp.14. 10.1186/1471-2229-7-14. hal-01738035 HAL Id: hal-01738035 https://hal.archives-ouvertes.fr/hal-01738035 Submitted on 20 Mar 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License BMC Plant Biology BioMed Central Research article Open Access Isolation and functional characterization of a cDNA coding a hydroxycinnamoyltransferase involved in phenylpropanoid biosynthesis in Cynara cardunculus -

Floral Structure and Dynamics of Nectar Production in Echinacea Pallida Var
Int. J. Plant Sci. 169(6):708–722. 2008. Ó 2008 by The University of Chicago. All rights reserved. 1058-5893/2008/16906-0002$15.00 DOI: 10.1086/533602 FLORAL STRUCTURE AND DYNAMICS OF NECTAR PRODUCTION IN ECHINACEA PALLIDA VAR. ANGUSTIFOLIA (ASTERACEAE) Tyler J. Wist and Arthur R. Davis1 Department of Biology, University of Saskatchewan, 112 Science Place, Saskatoon, Saskatchewan S7N 5E2, Canada The reproductive structure of the disk florets of Echinacea pallida var. angustifolia (Asteraceae) in relation to insect pollination was investigated using light, fluorescence, and scanning electron microscopy. The study of this self-incompatible species emphasized pollen production, pollen-stigma interactions, transmitting tissue, and vasculature within the style. Nectary structure and nectar production dynamics were also examined. Produced in the fused anther tubes, the trinucleate pollen with yellow pollenkitt was plentiful per floret, yielding a pollen : ovule ratio of 24,130. Encircling the style base at the ovary summit, the floral nectary pos- sessed modified stomata whose pores, as well as nonstomatal gaps in the epidermis, provided apoplastic pathways for nectar escape and reabsorption. Phloem alone supplied the gland interior, the sieve element– companion cell complexes reaching up to the nectary epidermis. Nectar was hexose dominant, its volume and nectar-sugar quantity per floret peaking on the afternoon of the first day of anthesis until the morning of the second day. Nectar production only occurred in half of the florets for 3 d, rarely for 5 d. Potential honey production from fields of this species was estimated at 2.1–11.9 kg/ha. Keywords: floral nectar, nectary, pollen-stigma interactions, pollination, style. -

Anatomy of the Underground Parts of Four Echinacea-Species and of Parthenium Integrifolium
Scientia Pharmaceutica (Sci. Pharm.) 69, 237-247 (2001) O Osterreichische Apotheker-Verlagsgesellschaft m.b.H., Wien, Printed in Austria Anatomy of the underground parts of four Echinacea-species and of Parthenium integrifolium R. Langer Institute of Pharmacognosy, University of Vienna Center of Pharmacy, Althanstrasse 14, A - 1090 Vienna, Austria Improved descriptions and detailed drawings of the most important anatomical characters of the roots of Echinacea purpurea (L.) MOENCH,E. angustifolia DC., E. pallida (NuTT.) NUTT.,and of Parfhenium integrifolium L. are presented. The anatomy of the rhizome of E. purpurea, which was detected in commercial samples, and of the root of E. atrorubens NUTT., another known adulteration for pharmaceutically used Echinacea-species, is documented for the first time. The possibilities and limitations of the identification by means of microscopy are discussed. The anatomical differences between the roots of E. angustifolia, E. pallida and E. atrorubens are not sufficient for differentiation, however, root and rhizome of E. purpurea and the root of Parthenium integrifolium appear well characterized. Because of the highly similar anatomy the microscopic proof of identity and purity of crude drugs of Echinacea must be done with uncomminuted material and the examination of cross sections. (Keywords: Echinacea angustifolia, Echinacea atrorubens, Echinacea pallida, Echinacea purpurea, Parthenium integrifolium, Asteraceae, microscopy, anatomy, identification) 1. Introduction The first, and for a long period only, detailed anatomical descriptions of the underground parts of Echinacea were published at the beginning of the last century', '. Due to later changes in the taxonomy within the genus Echinacea, unfortunately the plant sources for these descriptions remain unclear. The increasing interest in Echinacea and the adulterations that had been observed frequently caused Heubl et aL3 in the late eighties to examine the roots of E. -

Natural Medications for Psychiatric Disorders
Natural Medications for Psychiatric Disorders David Mischoulon, MD, PhD Director Depression Clinical and Research Program Massachusetts General Hospital Associate Professor of Psychiatry Harvard Medical School www.mghcme.org Disclosures • My spouse/partner and I have the following relevant financial relationship with a commercial interest to disclose: –Research support from Nordic Naturals –Unpaid consulting for Pharmavite, LLC, and Gnosis, Inc. –Royalties from Lippincott Williams & Wilkins for published book “Natural Medications for Psychiatric Disorders: Considering the Alternatives” www.mghcme.org 2 Objectives • To understand the evidence base for efficacy of natural therapies in psychiatry • To identify the risks and benefits of various natural treatments in psychiatry • To be able to educate patients in purchasing natural products in both over- the-counter and prescription forms www.mghcme.org Pros and Cons of Natural Remedies • In 2007, 38% of adults and 12% of children used CAM practices and products in the past year (NIH, 2010) – about $33.9 billion out-of-pocket cost • Easy access, good tolerability • Used by many who don’t respond to standard therapies • Limited research/systematic studies • “Natural” does NOT mean “safe” • Toxicity, adverse effects, interactions • Different preparations/purity • Insurance does not cover them www.mghcme.org 5 St. John’s Wort (SJW, Hypericum Perforatum) www.mghcme.org 6 St John’s Wort • About 40 published trials; many comparisons with TCAs and SSRIs; various systematic reviews and meta-analyses -

2016 What’S Hot 2016
OCTOBER 4-8 R Expo Hall October 6 & 7 2016 WHAT’S HOT 2016 THE WORLD'S LEADING INGREDIENT AND SOLUTIONS TRADESHOW WHERE SCIENCE & STRATEGY INTERSECT. WHAT’S HOT at SupplySide West TABLE OF CONTENTS A SPECIAL ALL-DIGITAL ISSUE October 2016 3 VIEWPOINT 4 AGENDA-AT-A-GLANCE 8 WHAT’S NEW IN CONTENT 9 SPORTS NUTRITION 10 SUPPLEMENTS & BEAUTY TABLE OF CONTENTS TABLE 11 LEGAL 12 FOOD & BEVERAGE 13 TARGETED CONTENT OPPORTUNITIES 16 SUPPLYSIDE CENTRAL 17 EDITOR’S CHOICE AWARDS 19 SURVIVAL KIT 20 FIRST-TIME ATTENDEE RECEPTION 22 EXHIBITOR NEWS 31 SUPPLYSIDE & VITAFOODS GLOBAL STOREFRONTS 32 HOT PRODUCTS Copyright © 2016 Informa Exhibitions LLC. All rights reserved. The publisher reserves the right to accept or reject any advertising or editorial material. Advertisers, and/or their agents, assume the responsibility for all content of published advertisements and assume responsibility for any claims against the publisher based on the advertisement. Editorial contributors assume responsibility for their published works and assume responsibility for any claims against the publisher based on the published work. Editorial content may not necessarily reflect the views of the publisher. Materials contained on this site may not be reproduced, modified, distributed, republished or hosted (either directly or by linking) without our prior written permission. You may not alter or remove any trademark, copyright or other notice from copies of content. You may, however, download material from the site (one machine readable copy and one print copy per page) for your personal, noncommercial use only. We reserve all rights in and title to all material downloaded. All items submitted to SUPPLYSIDE become the sole property of Informa Exhibitions LLC.