Methanol Health and Safety Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bromomethane CAS #: 74-83-9 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015
CHEMICAL UPDATE WORKSHEET Chemical Name: Bromomethane CAS #: 74-83-9 Revised By: RRD Toxicology Unit Revision Date: August 14, 2015 (A) Chemical-Physical Properties Part 201 Value Updated Value Reference Source Comments Molecular Weight (g/mol) 94.94 94.94 EPI EXP Physical State at ambient temp Liquid Gas MDEQ Melting Point (˚C) 179 -93.70 EPI EXP Boiling Point (˚C) 3.5 3.50 EPI EXP Solubility (ug/L) 1.45E+7 1.52E+07 EPI EXP Vapor Pressure (mmHg at 25˚C) 1672 1.62E+03 EPI EXP HLC (atm-m³/mol at 25˚C) 1.42E-2 7.34E-03 EPI EXP Log Kow (log P; octanol-water) 1.18 1.19 EPI EXP Koc (organic carbon; L/Kg) 14.5 13.22 EPI EST Ionizing Koc (L/kg) NR NA NA Diffusivity in Air (Di; cm2/s) 0.08 1.00E-01 W9 EST Diffusivity in Water (Dw; cm2/s) 8.0E-6 1.3468E-05 W9 EST CHEMICAL UPDATE WORKSHEET Bromomethane (74-83-9) Part 201 Value Updated Value Reference Source Comments Soil Water Partition Coefficient NR NR NA NA (Kd; inorganics) Flash Point (˚C) NA 194 PC EXP Lower Explosivity Level (LEL; 0.1 0.1 CRC EXP unit less) Critical Temperature (K) 467.00 EPA2004 EXP Enthalpy of Vaporization 5.71E+03 EPA2004 EXP (cal/mol) Density (g/mL, g/cm3) 1.6755 CRC EXP EMSOFT Flux Residential 2 m 2.69E-05 2.80E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Residential 5 m 6.53E-05 6.86E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Nonresidential 2 m 3.83E-05 4.47E-05 EMSOFT EST (mg/day/cm2) EMSOFT Flux Nonresidential 5 m 9.24E-05 1.09E-04 EMSOFT EST (mg/day/cm2) 2 CHEMICAL UPDATE WORKSHEET Bromomethane (74-83-9) (B) Toxicity Values/Benchmarks Source/Reference/ Comments/Notes Part 201 Value Updated Value Date /Issues Reference Dose 1.4E-3 2.0E-2 OPP, 2013 (RfD) (mg/kg/day) Rat subchronic Tier 1 Source: Complete gavage study EPA-OPP: (Danse et al., Basis: OPP is the more current than IRIS, PPRTV and ATSDR. -

SAFETY DATA SHEET Bromomethane (R40 B1) SECTION 1
SAFETY DATA SHEET Bromomethane (R40 B1) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021848 Last revised date: 02.02.2017 1/17 SECTION 1: Identification of the substance/mixture and of the company/undertaking 1.1 Product identifier Product name: Bromomethane (R40 B1) Additional identification Chemical name: Bromomethane Chemical formula: CH3Br INDEX No. 602-002-00-2 CAS-No. 74-83-9 EC No. 200-813-2 REACH Registration No. Not available. 1.2 Relevant identified uses of the substance or mixture and uses advised against Identified uses: Industrial and professional. Perform risk assessment prior to use. Using gas alone or in mixtures for the calibration of analysis equipment. Using gas as feedstock in chemical processes. Formulation of mixtures with gas in pressure receptacles. Uses advised against Consumer use. 1.3 Details of the supplier of the safety data sheet Supplier Linde Gas GmbH Telephone: +43 50 4273 Carl-von-Linde-Platz 1 A-4651 Stadl-Paura E-mail: [email protected] 1.4 Emergency telephone number: Emergency number Linde: + 43 50 4273 (during business hours), Poisoning Information Center: +43 1 406 43 43 SDS_AT - 000010021848 SAFETY DATA SHEET Bromomethane (R40 B1) Issue Date: 16.01.2013 Version: 1.0 SDS No.: 000010021848 Last revised date: 02.02.2017 2/17 SECTION 2: Hazards identification 2.1 Classification of the substance or mixture Classification according to Directive 67/548/EEC or 1999/45/EC as amended. T; R23/25 Xi; R36/37/38 Xn; R48/20 Muta. 3; R68 N; R50 N; R59 The full text for all R-phrases is displayed in section 16. -

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -

CAS REGISTRY: Finding CAS Registry Numbers
STN® Quick Reference Card CAS REGISTRYSM: Finding CAS Registry Numbers® When you know the Substance Name Field Use Example /CN Use when you know the complete => E BENZOIC ACID/CN Chemical substance name. EXPAND first to => E “BICYCLO(2.2.1)HEPTANE”/CN Name determine if the name is in the database. Basic Index Use when you know segments of the => S FLUOROMETHYL name or don’t know how the name => S “2,2’” (W) BIPYRID? segments go together. => S PYRIDINE (XW) DICARBOXY? /CNS Use when you want to search for a => S ?MYCIN?/CNS Chemical Name character string embedded in a trade => S ?QUAT/CNS Segments name. Use left and right truncation to search for an embedded character string. Use when you want to require that a => S CHLOROPHENYL/CNS name segment is not part of a larger => S HEXANEDIOIC (XW) segment. DIMETHYL/CNS /HP Use when you want to restrict the search => S 1-PROPANOL/HP Heading to a Heading Parent from a CA index => S 2-PYRIDINECARBO?/HP Parent name. /INS.HP Use when you want to restrict the search => S (PYRIDINE (XW) Index Name to name segments from the Heading CARBONITRIL?)/INS.HP part of a CA index name. Segments - Parent => S FLUOROMETHYL/INS.HP Heading Parent /INS.NHP Use when you want to restrict the search => S MORPHOL?/INS.NHP Index Name to name segments from the non- => S FLUOROMETHYL/INS.NHP Segments - part of a CA index Heading-Parent Non-Heading name. Parent /ONS Use when you want to restrict the search => S VINCAM?/ONS Other Name to name segments from names other => S (INDOLE(XW)AMIN?)/ONS Segments than CA index names such as semi- systematic names, trade names, common names, etc. -

REGISTRY Database Summary Sheet (DBSS)
SM REGISTRY/ZREGISTRY (CAS REGISTRY ) Subject • All types of inorganic and organic substances, including alloys, coordination Coverage compounds, minerals, mixtures, polymers, salts, high throughput screening (HTS) compounds as well as nucleic acid and protein sequences • Substances included in REGISTRY meet the following criteria: - Identified by CAS as coming from a reputable source, including but not limited to patents, journals, chemical catalogs, and selected substance collections on the web - Described in largely unambiguous terms - Characterized by physical methods or described in a patent document example or claim - Consistent with the laws of atomic covalent organization • Experimental and predicted property data and tags and spectra data File Type Numeric, Structure Features Alerts (SDIs) Biweekly In addition, SMARTracker, an automatic crossfile current-awareness search, (SDI XFILE) using a REGISTRY search profile in CA, HCA, ZCA, CAplus, HCAplus or ZCAplus may be run weekly or biweekly (weekly is the default). CAS Registry Keep & Share SLART Number® Identifiers ® Learning Database STN Easy Structures Record • CAS Registry Numbers • Experimental and predicted property Content • CA index names and commonly used data and tags chemical names and trade names • Spectra • Molecular formulas • List of databases and regulatory listings • Structure diagrams containing information on the substances • Sequence data • Super roles and document type SM • Alloy composition tables information from CAplus • Classes for polymers • Displayable information for 10 most recent references for substances • Ring analysis data File Size More than 140 million organic and inorganic substances; more than 71.1 million sequences (4/18) Coverage Early 1800s to the present Updates Daily Language English Database Chemical Abstracts Service Producer 2540 Olentangy River Road P.O. -

Methanol Interim AEGL Document
INTERIM 1: 1/2003 INTERIM 2: 2/2005 INTERIM ACUTE EXPOSURE GUIDELINE LEVELS (AEGLs) METHANOL (CAS Reg. No. 67-56-1) For NAS/COT Subcommittee for AEGLs February 2005 METHANOL Interim 2: 2/2005 PREFACE Under the authority of the Federal Advisory Committee Act (FACA) P. L. 92-463 of 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances (NAC/AEGL Committee) has been established to identify, review and interpret relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic chemicals. AEGLs represent threshold exposure limits for the general public and are applicable to emergency exposure periods ranging from 10 minutes to 8 hours. AEGL-2 and AEGL-3 levels, and AEGL-1 levels as appropriate, will be developed for each of five exposure periods (10 and 30 minutes, 1 hour, 4 hours, and 8 hours) and will be distinguished by varying degrees of severity of toxic effects. It is believed that the recommended exposure levels are applicable to the general population including infants and children, and other individuals who may be sensitive or susceptible. The three AEGLs have been defined as follows: AEGL-1 is the airborne concentration (expressed as ppm or mg/m;) of a substance above which it is predicted that the general population, including susceptible individuals, could experience notable discomfort, irritation, or certain asymptomatic, non-sensory effects. However, the effects are not disabling and are transient and reversible upon cessation of exposure. AEGL-2 is the airborne concentration (expressed as ppm or mg/m;) of a substance above which it is predicted that the general population, including susceptible individuals, could experience irreversible or other serious, long-lasting adverse health effects, or an impaired ability to escape. -

RR Program's RCL Spreadsheet Update
RR Program’s RCL Spreadsheet Update March 2017 RR Program RCL Spreadsheet Update DNR-RR-052e The Wisconsin DNR Remediation and Redevelopment Program (RR) has updated the numerical soil standards in the August 2015 DNR-RR- 052b RR spreadsheet of residual contaminant levels (RCLs). The RCLs were determined using the U.S. EPA RSL web- calculator by accepting EPA exposure defaults, with the exception of using Chicago, IL, for the climatic zone. This documentThe U.S. provides EPA updateda summary its Regionalof changes Screening to the direct-contact Level (RSL) RCLs website (DC-RCLs) in June that2015. are To now reflect in the that March 2017 spreadsheet.update, the The Wisconsin last page ofDNR this updated document the has numerical the EPA exposuresoil standards, parameter or residual values usedcontaminant in the RCL levels calculations. (RCLs), in the Remediation and Redevelopment program’s spreadsheet of RCLs. This document The providesU.S. EPA a RSL summary web-calculator of the updates has been incorporated recently updated in the Julyso that 2015 the spreadsheet.most up-to-date There toxicity were values no changes for chemi - cals madewere certainlyto the groundwater used in the RCLs,RCL calculations. but there are However, many changes it is important in the industrial to note that and the non-industrial web-calculator direct is only a subpartcontact of the (DC) full RCLsEPA RSL worksheets. webpage, Tables and that 1 andthe other 2 of thissubparts document that will summarize have important the DC-RCL explanatory changes text, generic tablesfrom and the references previous have spreadsheet yet to be (Januaryupdated. -

A High Volume Sampling System for Isotope Determination of Volatile Halocarbons and Hydrocarbons
Atmos. Meas. Tech., 4, 2073–2086, 2011 www.atmos-meas-tech.net/4/2073/2011/ Atmospheric doi:10.5194/amt-4-2073-2011 Measurement © Author(s) 2011. CC Attribution 3.0 License. Techniques A high volume sampling system for isotope determination of volatile halocarbons and hydrocarbons E. Bahlmann, I. Weinberg, R. Seifert, C. Tubbesing, and W. Michaelis Institute for Biogeochemistry and Marine Chemistry, Hamburg, Germany Received: 15 March 2011 – Published in Atmos. Meas. Tech. Discuss.: 8 April 2011 Revised: 31 August 2011 – Accepted: 7 September 2011 – Published: 4 October 2011 Abstract. The isotopic composition of volatile organic com- 1 Introduction pounds (VOCs) can provide valuable information on their sources and fate not deducible from mixing ratios alone. Compound specific isotope ratio mass spectrometry In particular the reported carbon stable isotope ratios of (CSIRMS) of non methane volatile organic compounds chloromethane and bromomethane from different sources (NMVOCs) emerged as a powerful tool to distinguish dif- 13 cover a δ C-range of almost 100 ‰ making isotope ra- ferent sources and to provide information on sinks (Rudolph tios a very promising tool for studying the biogeochemistry et al., 1997; Rudolph and Czuba, 2000; Tsunogai et al., of these compounds. So far, the determination of the iso- 1999; Bill et al., 2002; Thompson et al., 2002; Goldstein and topic composition of C1 and C2 halocarbons others than Shaw, 2003 and references therein; Archbold et al., 2005; chloromethane is hampered by their low mixing ratios. Redeker -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -

Methanol: Toxicological Overview
Methanol Toxicological Overview Key Points Kinetics and metabolism readily absorbed by all routes and distributed in the body water undergoes extensive metabolism, but small quantities are excreted unchanged by the lungs and in the urine Health effects of acute exposure methanol is toxic following ingestion, inhalation or dermal exposure exposure may initially result in CNS depression, followed by an asymptomatic latent period metabolic acidosis and ocular toxicity, which may result in blindness, are subsequent manifestations of toxicity coma and death may occur following substantial exposures long-term effects may include blindness and, following more substantial exposures, permanent damage to the CNS Health effects of chronic exposure long-term inhalation exposure to methanol has resulted in headaches and eye irritation in workers methanol is considered not to be a mutagen or carcinogen in humans methanol is considered not to be a reproductive toxicant in humans PHE publications gateway number: 2014790 Published: August 2015 Compendium of Chemical Hazards: Methanol Summary of Health Effects Methanol may be acutely toxic following inhalation, oral or dermal exposure. Acute methanol toxicity often follows a characteristic series of features; initially central nervous system (CNS) depression and gastrointestinal tract (GI) irritation may be observed. This is typically followed by a latent period of varying duration from 12–24 hours and occasionally up to 48 hours. Subsequently, a severe metabolic acidosis develops with nausea, vomiting and headache. Ocular toxicity ranges from photophobia and misty or blurred vision to markedly reduced visual acuity and complete blindness following high levels of exposure. Ingestion of as little as 4–10 mL of methanol in adults may cause permanent damage. -
Toxicological Profile for Bromomethane
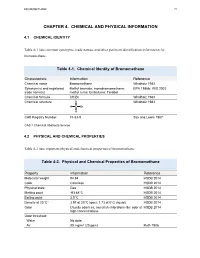
BROMOMETHANE 72 CHAPTER 4. CHEMICAL AND PHYSICAL INFORMATION 4.1 CHEMICAL IDENTITY Table 4-1 lists common synonyms, trade names, and other pertinent identification information for bromomethane. Table 4-1. Chemical Identity of Bromomethane Characteristic Information Reference Chemical name Bromomethane Windholz 1983 Synonym(s) and registered Methyl bromide; monobromomethane; EPA 1986b; IRIS 2002 trade name(s) methyl fume; Embafume; Terabol Chemical formula CH3Br Windholz 1983 Chemical structure H Windholz 1983 H C Br H CAS Registry Number 74-83-9 Sax and Lewis 1987 CAS = Chemical Abstracts Service 4.2 PHYSICAL AND CHEMICAL PROPERTIES Table 4-2 lists important physical and chemical properties of bromomethane. Table 4-2. Physical and Chemical Properties of Bromomethane Property Information Reference Molecular weight 94.94 HSDB 2014 Color Colorless HSDB 2014 Physical state Gas HSDB 2014 Melting point -93.68°C HSDB 2014 Boiling point 3.5°C HSDB 2014 Density at 20°Ca 3.97 at 20°C (gas); 1.73 at 0°C (liquid) HSDB 2014 Odor Usually odorless; sweetish chloroform-like odor at HSDB 2014 high concentrations Odor threshold: Water No data Air 80 mg/m3 (20 ppm) Ruth 1986 BROMOMETHANE 73 4. CHEMICAL AND PHYSICAL INFORMATION Table 4-2. Physical and Chemical Properties of Bromomethane Solubility: Water at 20°C 15.2 g/L at 25°C HSDB 2014 18.5 g/L at 20°C 13.4 g/L at 25°C Organic solvents Readily soluble in lower alcohols, ethers, esters, HSDB 2014 ketones, halogenated hydrocarbons, aromatic hydrocarbons, and carbon disulfide; freely soluble in benzene, carbon tetrachloride, and carbon disulfide; miscible in ethanol and chloroform Partition coefficients: Log Kow 1.19 HSDB 2014 Log Koc 0.95–1.3 Yates et al. -

METHANOL 6 (CAS Reg
1 INTERIM 1: 1/2003 2 INTERIM 2: 2/2005 3 INTERIM ACUTE EXPOSURE GUIDELINE LEVELS 4 (AEGLs) 5 METHANOL 6 (CAS Reg. No. 67-56-1) 7 For 8 NAS/COT Subcommittee for AEGLs 9 February 2005 METHANOL Interim 2: 2/2005 10 PREFACE 11 Under the authority of the Federal Advisory Committee Act (FACA) P. L. 92-463 of 1972, the 12 National Advisory Committee for Acute Exposure Guideline Levels for Hazardous Substances 13 (NAC/AEGL Committee) has been established to identify, review and interpret relevant toxicologic and 14 other scientific data and develop AEGLs for high priority, acutely toxic chemicals. 15 AEGLs represent threshold exposure limits for the general public and are applicable to emergency 16 exposure periods ranging from 10 minutes to 8 hours. AEGL-2 and AEGL-3 levels, and AEGL-1 levels as 17 appropriate, will be developed for each of five exposure periods (10 and 30 minutes, 1 hour, 4 hours, and 18 8 hours) and will be distinguished by varying degrees of severity of toxic effects. It is believed that the 19 recommended exposure levels are applicable to the general population including infants and children, and 20 other individuals who may be sensitive or susceptible. The three AEGLs have been defined as follows: 21 AEGL-1 is the airborne concentration (expressed as ppm or mg/m³) of a substance above which it 22 is predicted that the general population, including susceptible individuals, could experience notable 23 discomfort, irritation, or certain asymptomatic, non-sensory effects. However, the effects are not disabling 24 and are transient and reversible upon cessation of exposure.