한국 제주도에서 채집된 쥐치과(Monacanthidae) 어류 1미기록종, Cantherhines Multilineatus (Tanaka, 1918) 명세훈·김진구*·권혁준1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Marine Reconnaissance Survey of Proposed Sites for a Small Boat Harbor in Agat Bay, Guam
Ii MARINE RECONNAISSANCE SURVEY OF PROPOSED SITES FOR A SMALL BOAT HARBOR IN AGAT BAY, GUAM Mitchell 1. Chernin, Dennis R. Lassuy, Richard "E" Dickinson, and John W. Shepard II Submitted to !i U. S. Army Corps of Engineers Contract No. DACWB4-77-C-0061 I! I ~ Technical Report No. 39 University of Guam Marine Laboratory September 1977 , ,; , TABLE OF CONTENTS Page INTRODUCTION 1 METHODS 3 RESULTS AND DISCUSSION 5 Description of Study Sites 5 Macro-invertebrates 7 Marine Plants 13 Corals 22 Fishes 29 Currents 38 Water Quality 40 CONCLUSIONS AND RECOMMENDATIONS 47 LITERATURE CITED 49 APPENDIX 50 LIST OF FIGURES 1. General location map for the Agat Bay study areas. 2. Map indicating general topographical features at the Nimitz and Ta1eyfac study sites. 6 3. Map indicating general topographical features at the Bangi site. 17 4. Current movement along three transects at the Bangi site at low tide. 41 5. Current movement along two transects at the Bangi site at high tide. 42 6. Current movement on the Northern (Tr.IV) and Center (Tr.V) reef flats and Nimitz Channel during high tide. 43 7. Current movement on the Northern and Center reef flats and Nimitz Channel during a falling tide. 44 8. Current movement on the Taleyfac reef flat and Ta1eyfac Bay during a falling tide. 45 9. Current movement on the Ta1eyfac reef flat (Tr.VI) and Taleyfac Bay during a rising tide. 46 A-1. Location of two fish transects at the Gaan site 51 A-2. Density of fishes in each 2D-meter transect interval of Transect A (Fig. -

Redalyc.Feeding Ecology of the Planehead Filefish Stephanolepis
Revista de Biología Marina y Oceanografía ISSN: 0717-3326 [email protected] Universidad de Valparaíso Chile Mancera-Rodríguez, Néstor Javier; Castro-Hernández, José Juan Feeding ecology of the planehead filefish Stephanolepis hispidus (Pisces: Monacanthidae), in the Canary Islands area Revista de Biología Marina y Oceanografía, vol. 50, núm. 2, agosto, 2015, pp. 221-234 Universidad de Valparaíso Viña del Mar, Chile Available in: http://www.redalyc.org/articulo.oa?id=47941332002 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista de Biología Marina y Oceanografía Vol. 50, Nº2: 221-234, agosto 2015 DOI 10.4067/S0718-19572015000300002 ARTICLE Feeding ecology of the planehead filefish Stephanolepis hispidus (Pisces: Monacanthidae), in the Canary Islands area Ecología trófica de Stephanolepis hispidus (Pisces: Monacanthidae), en el área de las Islas Canarias Néstor Javier Mancera-Rodríguez1 and José Juan Castro-Hernández2 1Universidad Nacional de Colombia, Department of Forestry Sciences, Calle 59A No. 63-20, Bloque 20, oficina 211, Medellín, Colombia. [email protected] 2Universidad de Las Palmas de Gran Canaria, Department of Biology, Edf. Ciencias Básicas, Campus de Tafira, 35017, Las Palmas de Gran Canaria, Islas Canarias, España. [email protected] Resumen.- Se examinó la ecología alimentaria de Stephanolepis hispidus, en aguas de las Islas Canarias. El estudio se basó en el contenido estomacal de 823 ejemplares de 8,9 cm a 25,9 cm de longitud total (LT), capturados mensualmente en trampas para peces entre febrero de 1998 y junio de 1999. -
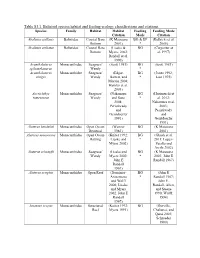
Table S3.1. Balistoid Species Habitat and Feeding Ecology Classifications and Citations
Table S3.1. Balistoid species habitat and feeding ecology classifications and citations. Species Family Habitat Habitat Feeding Feeding Mode Citation Mode Citation Abalistes stellaris Balistidae Coastal Bare (K Matsuura BG & EP (Kulbicki et al. Bottom 2001) * 2005) Abalistes stellatus Balistidae Coastal Bare (Lieske & BG (Carpenter et Bottom Myers, 2002; al. 1997) Randall et al. 1990) Acanthaluteres Monacanthidae Seagrass/ (Scott 1981) BG (Scott 1981) spilomelanurus Weedy * Acanthaluteres Monacanthidae Seagrass/ (Edgar, BG (Jones 1992; vittiger Weedy Barrett, and * Last 1975) Morton 2004; Hyndes et al. 2003) Acreichthys Monacanthidae Seagrass/ (Nakamura BG (Horinouchi et tomentosus Weedy and Sano * al. 2012; 2004; Nakamura et al. Peristiwady 2003; and Peristiwady Geistdoerfer and 1991) Geistdoerfer 1991) Aluterus heudeloti Monacanthidae Open Ocean (Wenner BG (K Matsuura Demersal 1983) 2001) Aluterus monoceros Monacanthidae Open Ocean (Kuiter 1992; BG (Ghosh et al. Rafting Lieske and * 2011; Lopez- Myers 2002) Peralta and Arcila 2002) Aluterus schoepfii Monacanthidae Seagrass/ (Lieske and BG (K Matsuura Weedy Myers 2002; * 2001; John E John E Randall 1967) Randall 1967) Aluterus scriptus Monacanthidae Open Reef (Dominici- BG (John E Arosemena * Randall 1967; and Wolff John E. 2006; Lieske Randall, Allen, and Myers and Steene 2002; John E 1990; Wulff, Randall 1994) 1967) Amanses scopas Monacanthidae Structured (Kuiter 1992; BG (Durville, Reef Myers 1991) Chabanet, and Quod 2003; Schroeder 1980) Balistapus Balistidae Structured (Hiatt and BG & EP (Hiatt and undulatus Reef Strasburg * Strasburg 1960; 1960; Lieske John E. Randall and Myers 1955; John E. 2002; Randall, Allen, McClanahan and Steene and Shafir 1990) 1990; John E. Randall, Allen, and Steene 1990) Balistes capriscus Balistidae Open Ocean (Longley BG & EP (Goldman, Pelagic 1941) * Glasgow, and Falk 2016; Lieske and Myers 2002; Vose and Nelson 1994) Balistes polylepis Balistidae Open Reef (Burgess et BG & EP (Abitia al. -

Annotated Checklist of the Fish Species (Pisces) of La Réunion, Including a Red List of Threatened and Declining Species
Stuttgarter Beiträge zur Naturkunde A, Neue Serie 2: 1–168; Stuttgart, 30.IV.2009. 1 Annotated checklist of the fish species (Pisces) of La Réunion, including a Red List of threatened and declining species RONALD FR ICKE , THIE rr Y MULOCHAU , PA tr ICK DU R VILLE , PASCALE CHABANE T , Emm ANUEL TESSIE R & YVES LE T OU R NEU R Abstract An annotated checklist of the fish species of La Réunion (southwestern Indian Ocean) comprises a total of 984 species in 164 families (including 16 species which are not native). 65 species (plus 16 introduced) occur in fresh- water, with the Gobiidae as the largest freshwater fish family. 165 species (plus 16 introduced) live in transitional waters. In marine habitats, 965 species (plus two introduced) are found, with the Labridae, Serranidae and Gobiidae being the largest families; 56.7 % of these species live in shallow coral reefs, 33.7 % inside the fringing reef, 28.0 % in shallow rocky reefs, 16.8 % on sand bottoms, 14.0 % in deep reefs, 11.9 % on the reef flat, and 11.1 % in estuaries. 63 species are first records for Réunion. Zoogeographically, 65 % of the fish fauna have a widespread Indo-Pacific distribution, while only 2.6 % are Mascarene endemics, and 0.7 % Réunion endemics. The classification of the following species is changed in the present paper: Anguilla labiata (Peters, 1852) [pre- viously A. bengalensis labiata]; Microphis millepunctatus (Kaup, 1856) [previously M. brachyurus millepunctatus]; Epinephelus oceanicus (Lacepède, 1802) [previously E. fasciatus (non Forsskål in Niebuhr, 1775)]; Ostorhinchus fasciatus (White, 1790) [previously Apogon fasciatus]; Mulloidichthys auriflamma (Forsskål in Niebuhr, 1775) [previously Mulloidichthys vanicolensis (non Valenciennes in Cuvier & Valenciennes, 1831)]; Stegastes luteobrun- neus (Smith, 1960) [previously S. -

Introduced Marine Species in Pago Pago Harbor, Fagatele Bay and the National Park Coast, American Samoa
INTRODUCED MARINE SPECIES IN PAGO PAGO HARBOR, FAGATELE BAY AND THE NATIONAL PARK COAST, AMERICAN SAMOA December 2003 COVER Typical views of benthic organisms from sampling areas (clockwise from upper left): Fouling organisms on debris at Pago Pago Harbor Dry Dock; Acropora hyacinthus tables in Fagetele Bay; Porites rus colonies in Fagasa Bay; Mixed branching and tabular Acropora in Vatia Bay INTRODUCED MARINE SPECIES IN PAGO PAGO HARBOR, FAGATELE BAY AND THE NATIONAL PARK COAST, AMERICAN SAMOA Final report prepared for the U.S. Fish and Wildlife Service, Fagetele Bay Marine Sanctuary, National Park of American Samoa and American Samoa Department of Marine and Natural Resources. S. L. Coles P. R. Reath P. A. Skelton V. Bonito R. C. DeFelice L. Basch Bishop Museum Pacific Biological Survey Bishop Museum Technical Report No 26 Honolulu Hawai‘i December 2003 Published by Bishop Museum Press 1525 Bernice Street Honolulu, Hawai‘i Copyright © 2003 Bishop Museum All Rights Reserved Printed in the United States of America ISSN 1085-455X Contribution No. 2003-007 to the Pacific Biological Survey EXECUTIVE SUMMARY The biological communities at ten sites around the Island of Tutuila, American Samoa were surveyed in October 2002 by a team of four investigators. Diving observations and collections of benthic observations using scuba and snorkel were made at six stations in Pago Pago Harbor, two stations in Fagatele Bay, and one station each in Vatia Bay and Fagasa Bay. The purpose of this survey was to determine the full complement of organisms greater than 0.5 mm in size, including benthic algae, macroinvertebrates and fishes, occurring at each site, and to evaluate the presence and potential impact of nonindigenous (introduced) marine species. -

Marine and Estuarine Fish Fauna of Tamil Nadu, India
Proceedings of the International Academy of Ecology and Environmental Sciences, 2018, 8(4): 231-271 Article Marine and estuarine fish fauna of Tamil Nadu, India 1,2 3 1 1 H.S. Mogalekar , J. Canciyal , D.S. Patadia , C. Sudhan 1Fisheries College and Research Institute, Thoothukudi - 628 008, Tamil Nadu, India 2College of Fisheries, Dholi, Muzaffarpur - 843 121, Bihar, India 3Central Inland Fisheries Research Institute, Barrackpore, Kolkata - 700 120, West Bengal, India E-mail: [email protected] Received 20 June 2018; Accepted 25 July 2018; Published 1 December 2018 Abstract Varied marine and estuarine ecosystems of Tamil Nadu endowed with diverse fish fauna. A total of 1656 fish species under two classes, 40 orders, 191 families and 683 geranra reported from marine and estuarine waters of Tamil Nadu. In the checklist, 1075 fish species were primary marine water and remaining 581 species were diadromus. In total, 128 species were reported under class Elasmobranchii (11 orders, 36 families and 70 genera) and 1528 species under class Actinopterygii (29 orders, 155 families and 613 genera). The top five order with diverse species composition were Perciformes (932 species; 56.29% of the total fauna), Tetraodontiformes (99 species), Pleuronectiforms (77 species), Clupeiformes (72 species) and Scorpaeniformes (69 species). At the family level, the Gobiidae has the greatest number of species (86 species), followed by the Carangidae (65 species), Labridae (64 species) and Serranidae (63 species). Fishery status assessment revealed existence of 1029 species worth for capture fishery, 425 species worth for aquarium fishery, 84 species worth for culture fishery, 242 species worth for sport fishery and 60 species worth for bait fishery. -

Tonga SUMA Report
BIOPHYSICALLY SPECIAL, UNIQUE MARINE AREAS OF TONGA EFFECTIVE MANAGEMENT Marine and coastal ecosystems of the Pacific Ocean provide benefits for all people in and beyond the region. To better understand and improve the effective management of these values on the ground, Pacific Island Countries are increasingly building institutional and personal capacities for Blue Planning. But there is no need to reinvent the wheel, when learning from experiences of centuries of traditional management in Pacific Island Countries. Coupled with scientific approaches these experiences can strengthen effective management of the region’s rich natural capital, if lessons learnt are shared. The MACBIO project collaborates with national and regional stakeholders towards documenting effective approaches to sustainable marine resource management and conservation. The project encourages and supports stakeholders to share tried and tested concepts and instruments more widely throughout partner countries and the Oceania region. This report outlines the process undertaken to define and describe the special, unique marine areas of Tonga. These special, unique marine areas provide an important input to decisions about, for example, permits, licences, EIAs and where to place different types of marine protected areas, locally managed marine areas and Community Conservation Areas in Tonga. For a copy of all reports and communication material please visit www.macbio-pacific.info. MARINE ECOSYSTEM MARINE SPATIAL PLANNING EFFECTIVE MANAGEMENT SERVICE VALUATION BIOPHYSICALLY SPECIAL, UNIQUE MARINE AREAS OF TONGA AUTHORS: Ceccarelli DM1, Wendt H2, Matoto AL3, Fonua E3, Fernandes L2 SUGGESTED CITATION: Ceccarelli DM, Wendt H, Matoto AL, Fonua E and Fernandes L (2017) Biophysically special, unique marine areas of Tonga. MACBIO (GIZ, IUCN, SPREP), Suva. -

View/Download
TETRAODONTIFORMES (part 2) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 1.0 - 30 Nov. 2020 Order TETRAODONTIFORMES (part 2 of 2) Suborder MOLOIDEI Family MOLIDAE Molas or Ocean Sunfishes 3 genera · 5 species Masturus Gill 1884 mast-, mastoid; oura, tail, referring to caudal fin (clavus) “extended backwards at the subaxial or submedian rays, and assuming a mastoid shape” Masturus lanceolatus (Liénard 1840) lanceolate, referring to shape of clavus (where dorsal and anal fins merge), forming a tail-like triangular lobe Mola Koelreuter 1766 millstone, referring to its somewhat circular shape (not tautonymous with Tetraodon mola Linnaeus 1758 since Koelreuter proposed a new species, M. aculeatus, actually a juvenile M. mola) Mola alexandrini (Ranzani 1839) in honor of Antonio Alessandrini (1786-1861, note latinization of name), Italian physician and anatomist, author of a detailed anatomical study of Mola gills published later that year [previously known as M. ramsayi] Mola mola (Linnaeus 1758) millstone, referring to its somewhat circular shape Mola tecta Nyegaard, Sawai, Gemmell, Gillum, Loneragan, Yamanoue & Stewart 2017 disguised or hidden, referring to how this species “evaded discovery for nearly three centuries, despite the keen interest among early sunfish taxonomists and the continued attention these curious fish receive” Ranzania Nardo 1840 -ia, belonging to: Camillo Ranzani (1775-1841), priest, naturalist and director of the Museum of Natural History of Bologna, for being the first to recognize Molidae as a distinct family [although authorship of family dates to Bonaparte 1835], and for “many other titles of merit in various branches of zoology” (translation) Ranzania laevis (Pennant 1776) smooth, referring to smooth skin covered with small, hard, hexagonal plates Mola alexandrini. -

Reproductive Behavior of the Honeycomb Leatherjacket
Japan. J. Ichthyol. 魚 類 学 雑 誌 41(1): 80-83, 1994 41(1): 80-83, 1 9 9 4 Materials andmethods Reproductive Behavior of the Honeycomb Leatherjacket, Cantherhines pardalis Underwater observations were carried out at a (Monacanthidae), at Kashiwajima, Japan rocky reef off Kashiwajima, southern Shikoku, Japan (32•‹46'N, 132•‹37'E) from June 18 to August 17, Hiroshi Kawasel and Akinobu Nakazono 1991. Kashiwajima is almost at the northern distri- bution limit of Cantherhines pardalis. Although not Department of Fisheries, Faculty of Agriculture , rare, the species was infrequent at Kashiwajima, as Kyushu University,6-10-1 Hakozaki, at Iriomotejima, Kuroshima, Akajima and Sesoko- Higashi-ku,Fukuoka 812, Jap an jima, Ryukyu Islands, Japan (24-27°N), due to its Present address:Natural Historymuseum 1and Institute , cryptic and wary nature (Kawase, personal observa- Chiba, 955-2 Aoba-cho, Chuo-ku, Chiba 260, Japa n tion; See alsomyers, 1989). For the reason given (ReceivedNovember 24, 1993; in revisedform January 20, above, C. pardalis was followed for as long as pos- 1994; acceptedmarch 29, 1994 ) sible when encountered during a study on another filefish, Stephanolepis cirrhifer, in an area of 40m Monacanthid fishes inhabit both temperate and 30m at 10-13m depth, and also on the way •~to and tropical shallow waters. About 95 species are known from the study area and the shore (total observation world wide (Nelson, 1984), including 26 species in time; 300min). In the earlymorning of July 20, a Japan (Matsuura, 1984). Among them, Stephano- pair of C. pardalis, both about 180mm TL (total lepis cirrhifer, Thamnaconusmodestus and Aluterus length), were observed spawning on algae. -

Check List of Rocky Reef Associated of Rocky Reef Associated Fishes Of
Research Journal of Marine Sciences ____________________________________ ______ _______ ISSN 2321-1296 Vol. 7(1), 1-13, June (2019) Res. J. Marine Sci. Check list of rocky reef associated fishes of south Kerala coast, India Baiju P.T. 1* , Prabhakaran M.P. 2, Benno Pereira F.G. 3 and V. Jayaprakas 4 1Department of Aquatic Biology and Fisheries, University of Kerala, Thiruvananthapuram 695581 Kerala, India 2Department of Aquatic Environment Management, School of Fisheries RM & HT, Kerala University of Fisheries and Ocean Studies, Panangad, Kochi – 682506, Kerala, India 3Department of Zoology, Uni versity of Kerala, Thiruvananthapuram -695581 Kerala, India 4Amity Thiruvananthapuram Center for Marine Science and Technology 3 Ravi Nagar, Ambalamukku, Peroorkada, P.O., Thiruvananthap uram- 695005, India [email protected] Available online at: www.isca.in, www.isca.me Received 25 th November 2018, revised 21 st March 2019, accepted 28 th April 2019 Abstract In this manuscript a checklist of 232 species of rocky reef associated fishes,a least documented area of tropical ichthyofauna recorded from the rocky reefs of south Kerala coast of the Indian Subcontinent. This 232 fish species were reported in the present study belong to 2 Classes, 16 Orders, 62 Families and 114Genera. Among the 62 Families recorded in the study, Pomacentridae the found as most specious family with 20 species of fishes belongs to 7 genera and followed by Labridae (18), Lutjanidae (15), Chaetodontidae (11), Apogonidae (10), Acanthuridae (11), Muraenidae (9), Serranida e (9), Scorpaenidae (8), Blenniidae (8), Haemulidae (6) , Mullidae (6), Holocentridae(5), Carangidae (5), Gobiidae(5) and Balistidae (5). Among the listed species, 141 species records asLeast Concern, followed by Not Included 77 species, Data Deficient 8 spe cies, Near Threatened 5 species, and Endangered and Vulnerable categories are 1 on each. -

Feeding Ecology of the Planehead Filefish Stephanolepis Hispidus
Revista de Biología Marina y Oceanografía Vol. 50, Nº2: 221-234, agosto 2015 DOI 10.4067/S0718-19572015000300002 ARTICLE Feeding ecology of the planehead filefish Stephanolepis hispidus (Pisces: Monacanthidae), in the Canary Islands area Ecología trófica de Stephanolepis hispidus (Pisces: Monacanthidae), en el área de las Islas Canarias Néstor Javier Mancera-Rodríguez1 and José Juan Castro-Hernández2 1Universidad Nacional de Colombia, Department of Forestry Sciences, Calle 59A No. 63-20, Bloque 20, oficina 211, Medellín, Colombia. [email protected] 2Universidad de Las Palmas de Gran Canaria, Department of Biology, Edf. Ciencias Básicas, Campus de Tafira, 35017, Las Palmas de Gran Canaria, Islas Canarias, España. [email protected] Resumen.- Se examinó la ecología alimentaria de Stephanolepis hispidus, en aguas de las Islas Canarias. El estudio se basó en el contenido estomacal de 823 ejemplares de 8,9 cm a 25,9 cm de longitud total (LT), capturados mensualmente en trampas para peces entre febrero de 1998 y junio de 1999. Aproximadamente el 27,2% de los peces mostraron estómagos vacíos. Esta proporción varió significativamente entre los sexos, pero no entre las clases de tamaño o las estaciones evaluadas. La dieta de Stephanolepis hispidus se compone principalmente por hidrozoos, anfípodos, equinodermos y algas. Gastrópodos, decápodos y lamelibranquios fueron presas secundarias. De acuerdo con el cambio de la ontogenia, los individuos de pequeño tamaño (<12,9 cm LT) se alimentan principalmente de pequeños crustáceos (anfípodos e hidrozoos), mientras que los especímenes de gran tamaño consumen equinoideos, algas, crustáceos y lamelibranquios. Hidrozoos y algas son más importantes en la dieta durante la primavera, y los anfípodos fueron más importantes en invierno. -

Checklist of the Coral Fish Fauna of Xisha Islands, China
Biodiversity Data Journal 9: e63945 doi: 10.3897/BDJ.9.e63945 Taxonomic Paper Checklist of the coral fish fauna of Xisha Islands, China Shuting Qiu‡, Bin Chen ‡,§, Jianguo Du‡,§, Kar-Hoe Loh |, Jianji Liao‡¶, Xinming Liu , Wen Yang‡ ‡ Third Institute of Oceanography, Ministry of Natural Resources, Xiamen, China § Fujian Provincial Key Laboratory ofMarine Ecological Conservation and Restoration, Xiamen, China | Institute of Ocean and Earth Sciences, University of Malaya, Kuala Lumpur, Malaysia ¶ Guangxi University of Chinese Medicine, Guangxi, China Corresponding author: Jianguo Du ([email protected]) Academic editor: Yahui Zhao Received: 04 Feb 2021 | Accepted: 01 Mar 2021 | Published: 08 Mar 2021 Citation: Qiu S, Chen B, Du J, Loh K-H, Liao J, Liu X, Yang W (2021) Checklist of the coral fish fauna of Xisha Islands, China. Biodiversity Data Journal 9: e63945. https://doi.org/10.3897/BDJ.9.e63945 Abstract Background The Xisha Islands are composed of the Yongle Islands and the Xuande Islands in Hainan Province, China. It has one of the highest species diversity in the world and is also a typical oceanic distribution area of coral reefs globally. The ichthyofauna of the Xisha Islands were recorded by underwater visual census in May 2019 and July 2020. The survey data were combined with previous records of species into the checklist of the Xisha Islands presented herein. A total of 691 species, belonging to 24 orders and 97 families, was recorded. The major families were Labridae, Pomacentridae, Serranidae, Chaetodontidae, Hexanchidae, Lutjanidae, Scaridae, Gobiidae, Scorpaenidae and Carangidae. In this study, the Coral Fish iversity Index (CFDI) of six families (Chaetodontidae, Pomacanthidae, Pomacentridae, Labridae, Scaridae and Acanthuridae) was 229, indicating 756 coral fishes.