On the Presence of Cantherhines Macrocerus (Hollard, 1853) in the Principe Island (Gulf of Guinea)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Survey of the Order Tetraodontiformes on Coral Reef Habitats in Southeast Florida
Nova Southeastern University NSUWorks HCNSO Student Capstones HCNSO Student Work 4-28-2020 A Survey of the Order Tetraodontiformes on Coral Reef Habitats in Southeast Florida Anne C. Sevon Nova Southeastern University, [email protected] This document is a product of extensive research conducted at the Nova Southeastern University . For more information on research and degree programs at the NSU , please click here. Follow this and additional works at: https://nsuworks.nova.edu/cnso_stucap Part of the Marine Biology Commons, and the Oceanography and Atmospheric Sciences and Meteorology Commons Share Feedback About This Item NSUWorks Citation Anne C. Sevon. 2020. A Survey of the Order Tetraodontiformes on Coral Reef Habitats in Southeast Florida. Capstone. Nova Southeastern University. Retrieved from NSUWorks, . (350) https://nsuworks.nova.edu/cnso_stucap/350. This Capstone is brought to you by the HCNSO Student Work at NSUWorks. It has been accepted for inclusion in HCNSO Student Capstones by an authorized administrator of NSUWorks. For more information, please contact [email protected]. Capstone of Anne C. Sevon Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science M.S. Marine Environmental Sciences M.S. Coastal Zone Management Nova Southeastern University Halmos College of Natural Sciences and Oceanography April 2020 Approved: Capstone Committee Major Professor: Dr. Kirk Kilfoyle Committee Member: Dr. Bernhard Riegl This capstone is available at NSUWorks: https://nsuworks.nova.edu/cnso_stucap/350 HALMOS -

Marine Reconnaissance Survey of Proposed Sites for a Small Boat Harbor in Agat Bay, Guam
Ii MARINE RECONNAISSANCE SURVEY OF PROPOSED SITES FOR A SMALL BOAT HARBOR IN AGAT BAY, GUAM Mitchell 1. Chernin, Dennis R. Lassuy, Richard "E" Dickinson, and John W. Shepard II Submitted to !i U. S. Army Corps of Engineers Contract No. DACWB4-77-C-0061 I! I ~ Technical Report No. 39 University of Guam Marine Laboratory September 1977 , ,; , TABLE OF CONTENTS Page INTRODUCTION 1 METHODS 3 RESULTS AND DISCUSSION 5 Description of Study Sites 5 Macro-invertebrates 7 Marine Plants 13 Corals 22 Fishes 29 Currents 38 Water Quality 40 CONCLUSIONS AND RECOMMENDATIONS 47 LITERATURE CITED 49 APPENDIX 50 LIST OF FIGURES 1. General location map for the Agat Bay study areas. 2. Map indicating general topographical features at the Nimitz and Ta1eyfac study sites. 6 3. Map indicating general topographical features at the Bangi site. 17 4. Current movement along three transects at the Bangi site at low tide. 41 5. Current movement along two transects at the Bangi site at high tide. 42 6. Current movement on the Northern (Tr.IV) and Center (Tr.V) reef flats and Nimitz Channel during high tide. 43 7. Current movement on the Northern and Center reef flats and Nimitz Channel during a falling tide. 44 8. Current movement on the Taleyfac reef flat and Ta1eyfac Bay during a falling tide. 45 9. Current movement on the Ta1eyfac reef flat (Tr.VI) and Taleyfac Bay during a rising tide. 46 A-1. Location of two fish transects at the Gaan site 51 A-2. Density of fishes in each 2D-meter transect interval of Transect A (Fig. -

Andrew David Dorka Cobián Rojas Felicia Drummond Alain García Rodríguez
CUBA’S MESOPHOTIC CORAL REEFS Fish Photo Identification Guide ANDREW DAVID DORKA COBIÁN ROJAS FELICIA DRUMMOND ALAIN GARCÍA RODRÍGUEZ Edited by: John K. Reed Stephanie Farrington CUBA’S MESOPHOTIC CORAL REEFS Fish Photo Identification Guide ANDREW DAVID DORKA COBIÁN ROJAS FELICIA DRUMMOND ALAIN GARCÍA RODRÍGUEZ Edited by: John K. Reed Stephanie Farrington ACKNOWLEDGMENTS This research was supported by the NOAA Office of Ocean Exploration and Research under award number NA14OAR4320260 to the Cooperative Institute for Ocean Exploration, Research and Technology (CIOERT) at Harbor Branch Oceanographic Institute-Florida Atlantic University (HBOI-FAU), and by the NOAA Pacific Marine Environmental Laboratory under award number NA150AR4320064 to the Cooperative Institute for Marine and Atmospheric Studies (CIMAS) at the University of Miami. This expedition was conducted in support of the Joint Statement between the United States of America and the Republic of Cuba on Cooperation on Environmental Protection (November 24, 2015) and the Memorandum of Understanding between the United States National Oceanic and Atmospheric Administration, the U.S. National Park Service, and Cuba’s National Center for Protected Areas. We give special thanks to Carlos Díaz Maza (Director of the National Center of Protected Areas) and Ulises Fernández Gomez (International Relations Officer, Ministry of Science, Technology and Environment; CITMA) for assistance in securing the necessary permits to conduct the expedition and for their tremendous hospitality and logistical support in Cuba. We thank the Captain and crew of the University of Miami R/V F.G. Walton Smith and ROV operators Lance Horn and Jason White, University of North Carolina at Wilmington (UNCW-CIOERT), Undersea Vehicle Program for their excellent work at sea during the expedition. -

The Marine Biodiversity and Fisheries Catches of the Pitcairn Island Group
The Marine Biodiversity and Fisheries Catches of the Pitcairn Island Group THE MARINE BIODIVERSITY AND FISHERIES CATCHES OF THE PITCAIRN ISLAND GROUP M.L.D. Palomares, D. Chaitanya, S. Harper, D. Zeller and D. Pauly A report prepared for the Global Ocean Legacy project of the Pew Environment Group by the Sea Around Us Project Fisheries Centre The University of British Columbia 2202 Main Mall Vancouver, BC, Canada, V6T 1Z4 TABLE OF CONTENTS FOREWORD ................................................................................................................................................. 2 Daniel Pauly RECONSTRUCTION OF TOTAL MARINE FISHERIES CATCHES FOR THE PITCAIRN ISLANDS (1950-2009) ...................................................................................... 3 Devraj Chaitanya, Sarah Harper and Dirk Zeller DOCUMENTING THE MARINE BIODIVERSITY OF THE PITCAIRN ISLANDS THROUGH FISHBASE AND SEALIFEBASE ..................................................................................... 10 Maria Lourdes D. Palomares, Patricia M. Sorongon, Marianne Pan, Jennifer C. Espedido, Lealde U. Pacres, Arlene Chon and Ace Amarga APPENDICES ............................................................................................................................................... 23 APPENDIX 1: FAO AND RECONSTRUCTED CATCH DATA ......................................................................................... 23 APPENDIX 2: TOTAL RECONSTRUCTED CATCH BY MAJOR TAXA ............................................................................ -

Redalyc.Feeding Ecology of the Planehead Filefish Stephanolepis
Revista de Biología Marina y Oceanografía ISSN: 0717-3326 [email protected] Universidad de Valparaíso Chile Mancera-Rodríguez, Néstor Javier; Castro-Hernández, José Juan Feeding ecology of the planehead filefish Stephanolepis hispidus (Pisces: Monacanthidae), in the Canary Islands area Revista de Biología Marina y Oceanografía, vol. 50, núm. 2, agosto, 2015, pp. 221-234 Universidad de Valparaíso Viña del Mar, Chile Available in: http://www.redalyc.org/articulo.oa?id=47941332002 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Revista de Biología Marina y Oceanografía Vol. 50, Nº2: 221-234, agosto 2015 DOI 10.4067/S0718-19572015000300002 ARTICLE Feeding ecology of the planehead filefish Stephanolepis hispidus (Pisces: Monacanthidae), in the Canary Islands area Ecología trófica de Stephanolepis hispidus (Pisces: Monacanthidae), en el área de las Islas Canarias Néstor Javier Mancera-Rodríguez1 and José Juan Castro-Hernández2 1Universidad Nacional de Colombia, Department of Forestry Sciences, Calle 59A No. 63-20, Bloque 20, oficina 211, Medellín, Colombia. [email protected] 2Universidad de Las Palmas de Gran Canaria, Department of Biology, Edf. Ciencias Básicas, Campus de Tafira, 35017, Las Palmas de Gran Canaria, Islas Canarias, España. [email protected] Resumen.- Se examinó la ecología alimentaria de Stephanolepis hispidus, en aguas de las Islas Canarias. El estudio se basó en el contenido estomacal de 823 ejemplares de 8,9 cm a 25,9 cm de longitud total (LT), capturados mensualmente en trampas para peces entre febrero de 1998 y junio de 1999. -
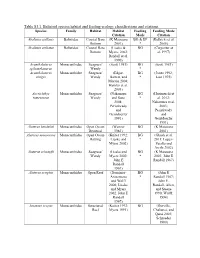
Table S3.1. Balistoid Species Habitat and Feeding Ecology Classifications and Citations
Table S3.1. Balistoid species habitat and feeding ecology classifications and citations. Species Family Habitat Habitat Feeding Feeding Mode Citation Mode Citation Abalistes stellaris Balistidae Coastal Bare (K Matsuura BG & EP (Kulbicki et al. Bottom 2001) * 2005) Abalistes stellatus Balistidae Coastal Bare (Lieske & BG (Carpenter et Bottom Myers, 2002; al. 1997) Randall et al. 1990) Acanthaluteres Monacanthidae Seagrass/ (Scott 1981) BG (Scott 1981) spilomelanurus Weedy * Acanthaluteres Monacanthidae Seagrass/ (Edgar, BG (Jones 1992; vittiger Weedy Barrett, and * Last 1975) Morton 2004; Hyndes et al. 2003) Acreichthys Monacanthidae Seagrass/ (Nakamura BG (Horinouchi et tomentosus Weedy and Sano * al. 2012; 2004; Nakamura et al. Peristiwady 2003; and Peristiwady Geistdoerfer and 1991) Geistdoerfer 1991) Aluterus heudeloti Monacanthidae Open Ocean (Wenner BG (K Matsuura Demersal 1983) 2001) Aluterus monoceros Monacanthidae Open Ocean (Kuiter 1992; BG (Ghosh et al. Rafting Lieske and * 2011; Lopez- Myers 2002) Peralta and Arcila 2002) Aluterus schoepfii Monacanthidae Seagrass/ (Lieske and BG (K Matsuura Weedy Myers 2002; * 2001; John E John E Randall 1967) Randall 1967) Aluterus scriptus Monacanthidae Open Reef (Dominici- BG (John E Arosemena * Randall 1967; and Wolff John E. 2006; Lieske Randall, Allen, and Myers and Steene 2002; John E 1990; Wulff, Randall 1994) 1967) Amanses scopas Monacanthidae Structured (Kuiter 1992; BG (Durville, Reef Myers 1991) Chabanet, and Quod 2003; Schroeder 1980) Balistapus Balistidae Structured (Hiatt and BG & EP (Hiatt and undulatus Reef Strasburg * Strasburg 1960; 1960; Lieske John E. Randall and Myers 1955; John E. 2002; Randall, Allen, McClanahan and Steene and Shafir 1990) 1990; John E. Randall, Allen, and Steene 1990) Balistes capriscus Balistidae Open Ocean (Longley BG & EP (Goldman, Pelagic 1941) * Glasgow, and Falk 2016; Lieske and Myers 2002; Vose and Nelson 1994) Balistes polylepis Balistidae Open Reef (Burgess et BG & EP (Abitia al. -

Annotated Checklist of the Fish Species (Pisces) of La Réunion, Including a Red List of Threatened and Declining Species
Stuttgarter Beiträge zur Naturkunde A, Neue Serie 2: 1–168; Stuttgart, 30.IV.2009. 1 Annotated checklist of the fish species (Pisces) of La Réunion, including a Red List of threatened and declining species RONALD FR ICKE , THIE rr Y MULOCHAU , PA tr ICK DU R VILLE , PASCALE CHABANE T , Emm ANUEL TESSIE R & YVES LE T OU R NEU R Abstract An annotated checklist of the fish species of La Réunion (southwestern Indian Ocean) comprises a total of 984 species in 164 families (including 16 species which are not native). 65 species (plus 16 introduced) occur in fresh- water, with the Gobiidae as the largest freshwater fish family. 165 species (plus 16 introduced) live in transitional waters. In marine habitats, 965 species (plus two introduced) are found, with the Labridae, Serranidae and Gobiidae being the largest families; 56.7 % of these species live in shallow coral reefs, 33.7 % inside the fringing reef, 28.0 % in shallow rocky reefs, 16.8 % on sand bottoms, 14.0 % in deep reefs, 11.9 % on the reef flat, and 11.1 % in estuaries. 63 species are first records for Réunion. Zoogeographically, 65 % of the fish fauna have a widespread Indo-Pacific distribution, while only 2.6 % are Mascarene endemics, and 0.7 % Réunion endemics. The classification of the following species is changed in the present paper: Anguilla labiata (Peters, 1852) [pre- viously A. bengalensis labiata]; Microphis millepunctatus (Kaup, 1856) [previously M. brachyurus millepunctatus]; Epinephelus oceanicus (Lacepède, 1802) [previously E. fasciatus (non Forsskål in Niebuhr, 1775)]; Ostorhinchus fasciatus (White, 1790) [previously Apogon fasciatus]; Mulloidichthys auriflamma (Forsskål in Niebuhr, 1775) [previously Mulloidichthys vanicolensis (non Valenciennes in Cuvier & Valenciennes, 1831)]; Stegastes luteobrun- neus (Smith, 1960) [previously S. -

MONACANTHIDAE Filefishes (Leatherjackets) by K
click for previous page 1970 Bony Fishes MONACANTHIDAE Filefishes (leatherjackets) by K. Matsuura, National Science Museum, Tokyo, Japan iagnostic characters: Small or medium-sized fishes, usually less than 20 cm (but up to 50 cm for some Dspecies of Aluterus), with deep, highly compressed bodies covered by thin but rough or shag- reen-like skin with innumerable minute scales not individually easily discernible to the unaided eye. Mouth small and usually more or less terminal or slightly supraterminal;teeth only moderately heavy,6 in an outer series in upper jaw and 6 or fewer in the lower. Gill opening a relatively short, vertical to oblique slit in front of pectoral-fin base, branchiostegal rays hidden beneath the skin. Two (sometimes 1) dor- sal-fin spines, second spine not more than 1/3 the length of first; first spine usually capable of being locked in an upright position of erection by the second; dorsal-, anal- and pectoral-fin rays unbranched; pelvic fin and spines rudimentary or absent, represented by a series of 3 or fewer pairs of enlarged scales encasing end of pelvis, or segments of indeterminate number, or entirely absent. Scales above pectoral-fin base unmodified, not forming a tympanum. Lateral line inconspicuous or only slightly apparent. Colour: variable, drab brown, grey, or greenish, but often with strikingly marked and vivid patterns. 1st spine prominent 2nd spine fin rays minute unbranched 6 outer teeth 6or fewer teeth restricted gill slit numerous minute encasing scales at scales end of pelvis Habitat, biology,and fisheries: Filefishes range in depth down to about 90 m.They are primarily benthic spe- cies living around coral and rocky reefs or on sand and mud bottoms and seagrass beds. -

Introduced Marine Species in Pago Pago Harbor, Fagatele Bay and the National Park Coast, American Samoa
INTRODUCED MARINE SPECIES IN PAGO PAGO HARBOR, FAGATELE BAY AND THE NATIONAL PARK COAST, AMERICAN SAMOA December 2003 COVER Typical views of benthic organisms from sampling areas (clockwise from upper left): Fouling organisms on debris at Pago Pago Harbor Dry Dock; Acropora hyacinthus tables in Fagetele Bay; Porites rus colonies in Fagasa Bay; Mixed branching and tabular Acropora in Vatia Bay INTRODUCED MARINE SPECIES IN PAGO PAGO HARBOR, FAGATELE BAY AND THE NATIONAL PARK COAST, AMERICAN SAMOA Final report prepared for the U.S. Fish and Wildlife Service, Fagetele Bay Marine Sanctuary, National Park of American Samoa and American Samoa Department of Marine and Natural Resources. S. L. Coles P. R. Reath P. A. Skelton V. Bonito R. C. DeFelice L. Basch Bishop Museum Pacific Biological Survey Bishop Museum Technical Report No 26 Honolulu Hawai‘i December 2003 Published by Bishop Museum Press 1525 Bernice Street Honolulu, Hawai‘i Copyright © 2003 Bishop Museum All Rights Reserved Printed in the United States of America ISSN 1085-455X Contribution No. 2003-007 to the Pacific Biological Survey EXECUTIVE SUMMARY The biological communities at ten sites around the Island of Tutuila, American Samoa were surveyed in October 2002 by a team of four investigators. Diving observations and collections of benthic observations using scuba and snorkel were made at six stations in Pago Pago Harbor, two stations in Fagatele Bay, and one station each in Vatia Bay and Fagasa Bay. The purpose of this survey was to determine the full complement of organisms greater than 0.5 mm in size, including benthic algae, macroinvertebrates and fishes, occurring at each site, and to evaluate the presence and potential impact of nonindigenous (introduced) marine species. -

61661147.Pdf
Resource Inventory of Marine and Estuarine Fishes of the West Coast and Alaska: A Checklist of North Pacific and Arctic Ocean Species from Baja California to the Alaska–Yukon Border OCS Study MMS 2005-030 and USGS/NBII 2005-001 Project Cooperation This research addressed an information need identified Milton S. Love by the USGS Western Fisheries Research Center and the Marine Science Institute University of California, Santa Barbara to the Department University of California of the Interior’s Minerals Management Service, Pacific Santa Barbara, CA 93106 OCS Region, Camarillo, California. The resource inventory [email protected] information was further supported by the USGS’s National www.id.ucsb.edu/lovelab Biological Information Infrastructure as part of its ongoing aquatic GAP project in Puget Sound, Washington. Catherine W. Mecklenburg T. Anthony Mecklenburg Report Availability Pt. Stephens Research Available for viewing and in PDF at: P. O. Box 210307 http://wfrc.usgs.gov Auke Bay, AK 99821 http://far.nbii.gov [email protected] http://www.id.ucsb.edu/lovelab Lyman K. Thorsteinson Printed copies available from: Western Fisheries Research Center Milton Love U. S. Geological Survey Marine Science Institute 6505 NE 65th St. University of California, Santa Barbara Seattle, WA 98115 Santa Barbara, CA 93106 [email protected] (805) 893-2935 June 2005 Lyman Thorsteinson Western Fisheries Research Center Much of the research was performed under a coopera- U. S. Geological Survey tive agreement between the USGS’s Western Fisheries -

Marine and Estuarine Fish Fauna of Tamil Nadu, India
Proceedings of the International Academy of Ecology and Environmental Sciences, 2018, 8(4): 231-271 Article Marine and estuarine fish fauna of Tamil Nadu, India 1,2 3 1 1 H.S. Mogalekar , J. Canciyal , D.S. Patadia , C. Sudhan 1Fisheries College and Research Institute, Thoothukudi - 628 008, Tamil Nadu, India 2College of Fisheries, Dholi, Muzaffarpur - 843 121, Bihar, India 3Central Inland Fisheries Research Institute, Barrackpore, Kolkata - 700 120, West Bengal, India E-mail: [email protected] Received 20 June 2018; Accepted 25 July 2018; Published 1 December 2018 Abstract Varied marine and estuarine ecosystems of Tamil Nadu endowed with diverse fish fauna. A total of 1656 fish species under two classes, 40 orders, 191 families and 683 geranra reported from marine and estuarine waters of Tamil Nadu. In the checklist, 1075 fish species were primary marine water and remaining 581 species were diadromus. In total, 128 species were reported under class Elasmobranchii (11 orders, 36 families and 70 genera) and 1528 species under class Actinopterygii (29 orders, 155 families and 613 genera). The top five order with diverse species composition were Perciformes (932 species; 56.29% of the total fauna), Tetraodontiformes (99 species), Pleuronectiforms (77 species), Clupeiformes (72 species) and Scorpaeniformes (69 species). At the family level, the Gobiidae has the greatest number of species (86 species), followed by the Carangidae (65 species), Labridae (64 species) and Serranidae (63 species). Fishery status assessment revealed existence of 1029 species worth for capture fishery, 425 species worth for aquarium fishery, 84 species worth for culture fishery, 242 species worth for sport fishery and 60 species worth for bait fishery. -

Evidence of the Role of Tropical Cyclone Ockhi Muhammed Zafar Iqbal A.N.1*, Gurudev Mali1, Raghavendra Dollin1
bioRxiv preprint doi: https://doi.org/10.1101/2021.06.28.450260; this version posted June 30, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. First report of Lactoria cornuta from the west coast of India – Evidence of the role of tropical cyclone Ockhi Muhammed Zafar Iqbal A.N.1*, Gurudev Mali1, Raghavendra Dollin1 1. Post Graduate Department of Zoology, Government First Grade College, Karwar, Karnataka, India. Corresponding Author Dr Muhammed Zafar Iqbal A.N. Post Graduate Department of Zoology, Government First Grade College, Karwar, Karnataka, India. Email: [email protected] Abstract India is known for its rich biodiversity and is fortunate to have several endemic species from different classes of vertebrates. India is home to 7.5% of the global fish diversity, with 91 endemic species of ray finned fishes, the actinopterygians. Some fish species have never been reported until recently, and one such example is the long-horned Cowfish (Ostracidae), best known for inhabiting only certain areas of the Indo-Pacific oceans. However, it has been reported recently in the Bay of Bengal, but never on the west coast of India. This is the first time this fish has been found on the west coast of India. Given its morphology, the migration seems highly improbable since it can only endure lethargic swimming. In this article, we have explored the role of other external forces that could have contributed to its journey to the west coast of India. As such, we recognize the role of Super Cyclone Ockhi as a vital force in determining the expansion of its range.