Solutions: Environmental Chemistry - a Global Perspective 4Th Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Development Team
Paper No: 16 Environmental Chemistry Module: 01 Environmental Concentration Units Development Team Prof. R.K. Kohli Principal Investigator & Prof. V.K. Garg & Prof. Ashok Dhawan Co- Principal Investigator Central University of Punjab, Bathinda Prof. K.S. Gupta Paper Coordinator University of Rajasthan, Jaipur Prof. K.S. Gupta Content Writer University of Rajasthan, Jaipur Content Reviewer Dr. V.K. Garg Central University of Punjab, Bathinda Anchor Institute Central University of Punjab 1 Environmental Chemistry Environmental Environmental Concentration Units Sciences Description of Module Subject Name Environmental Sciences Paper Name Environmental Chemistry Module Name/Title Environmental Concentration Units Module Id EVS/EC-XVI/01 Pre-requisites A basic knowledge of concentration units 1. To define exponents, prefixes and symbols based on SI units 2. To define molarity and molality 3. To define number density and mixing ratio 4. To define parts –per notation by volume Objectives 5. To define parts-per notation by mass by mass 6. To define mass by volume unit for trace gases in air 7. To define mass by volume unit for aqueous media 8. To convert one unit into another Keywords Environmental concentrations, parts- per notations, ppm, ppb, ppt, partial pressure 2 Environmental Chemistry Environmental Environmental Concentration Units Sciences Module 1: Environmental Concentration Units Contents 1. Introduction 2. Exponents 3. Environmental Concentration Units 4. Molarity, mol/L 5. Molality, mol/kg 6. Number Density (n) 7. Mixing Ratio 8. Parts-Per Notation by Volume 9. ppmv, ppbv and pptv 10. Parts-Per Notation by Mass by Mass. 11. Mass by Volume Unit for Trace Gases in Air: Microgram per Cubic Meter, µg/m3 12. -

Hohonu Volume 5 (PDF)
HOHONU 2007 VOLUME 5 A JOURNAL OF ACADEMIC WRITING This publication is available in alternate format upon request. TheUniversity of Hawai‘i is an Equal Opportunity Affirmative Action Institution. VOLUME 5 Hohonu 2 0 0 7 Academic Journal University of Hawai‘i at Hilo • Hawai‘i Community College Hohonu is publication funded by University of Hawai‘i at Hilo and Hawai‘i Community College student fees. All production and printing costs are administered by: University of Hawai‘i at Hilo/Hawai‘i Community College Board of Student Publications 200 W. Kawili Street Hilo, Hawai‘i 96720-4091 Phone: (808) 933-8823 Web: www.uhh.hawaii.edu/campuscenter/bosp All rights revert to the witers upon publication. All requests for reproduction and other propositions should be directed to writers. ii d d d d d d d d d d d d d d d d d d d d d d Table of Contents 1............................ A Fish in the Hand is Worth Two on the Net: Don’t Make me Think…different, by Piper Seldon 4..............................................................................................Abortion: Murder-Or Removal of Tissue?, by Dane Inouye 9...............................An Etymology of Four English Words, with Reference to both Grimm’s Law and Verner’s Law by Piper Seldon 11................................Artifacts and Native Burial Rights: Where do We Draw the Line?, by Jacqueline Van Blarcon 14..........................................................................................Ayahuasca: Earth’s Wisdom Revealed, by Jennifer Francisco 16......................................Beak of the Fish: What Cichlid Flocks Reveal About Speciation Processes, by Holly Jessop 26................................................................................. Climatic Effects of the 1815 Eruption of Tambora, by Jacob Smith 33...........................Columnar Joints: An Examination of Features, Formation and Cooling Models, by Mary Mathis 36.................... -

The Vertical Distribution of Soluble Gases in the Troposphere
UC Irvine UC Irvine Previously Published Works Title The vertical distribution of soluble gases in the troposphere Permalink https://escholarship.org/uc/item/7gc9g430 Journal Geophysical Research Letters, 2(8) ISSN 0094-8276 Authors Stedman, DH Chameides, WL Cicerone, RJ Publication Date 1975 DOI 10.1029/GL002i008p00333 License https://creativecommons.org/licenses/by/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Vol. 2 No. 8 GEOPHYSICAL RESEARCHLETTERS August 1975 THE VERTICAL DISTRIBUTION OF SOLUBLE GASES IN THE TROPOSPHERE D. H. Stedman, W. L. Chameides, and R. J. Cicerone Space Physics Research Laboratory Department of Atmospheric and Oceanic Science The University of Michigan, Ann Arbor, Michigan 48105 Abstrg.ct. The thermodynamic properties of sev- Their arg:uments were based on the existence of an eral water-soluble gases are reviewed to determine HC1-H20 d•mer, but we consider the conclusions in- the likely effect of the atmospheric water cycle dependeht of the existence of dimers. For in- on their vertical profiles. We find that gaseous stance, Chameides[1975] has argued that HNO3 HC1, HN03, and HBr are sufficiently soluble in should follow the water-vapor mixing ratio gradi- water to suggest that their vertical profiles in ent, based on the high solubility of the gas in the troposphere have a similar shape to that of liquid water. In this work, we generalize the water va•or. Thuswe predict that HC1,HN03, and moredetailed argumentsof Chameides[1975] and HBr exhibit a steep negative gradient with alti- suggest which species should exhibit this nega- tude roughly equal to the altitude gradient of tive gradient and which should not. -

Package 'Marelac'
Package ‘marelac’ February 20, 2020 Version 2.1.10 Title Tools for Aquatic Sciences Author Karline Soetaert [aut, cre], Thomas Petzoldt [aut], Filip Meysman [cph], Lorenz Meire [cph] Maintainer Karline Soetaert <[email protected]> Depends R (>= 3.2), shape Imports stats, seacarb Description Datasets, constants, conversion factors, and utilities for 'MArine', 'Riverine', 'Estuarine', 'LAcustrine' and 'Coastal' science. The package contains among others: (1) chemical and physical constants and datasets, e.g. atomic weights, gas constants, the earths bathymetry; (2) conversion factors (e.g. gram to mol to liter, barometric units, temperature, salinity); (3) physical functions, e.g. to estimate concentrations of conservative substances, gas transfer and diffusion coefficients, the Coriolis force and gravity; (4) thermophysical properties of the seawater, as from the UNESCO polynomial or from the more recent derivation based on a Gibbs function. License GPL (>= 2) LazyData yes Repository CRAN Repository/R-Forge/Project marelac Repository/R-Forge/Revision 205 Repository/R-Forge/DateTimeStamp 2020-02-11 22:10:45 Date/Publication 2020-02-20 18:50:04 UTC NeedsCompilation yes 1 2 R topics documented: R topics documented: marelac-package . .3 air_density . .5 air_spechum . .6 atmComp . .7 AtomicWeight . .8 Bathymetry . .9 Constants . 10 convert_p . 11 convert_RtoS . 11 convert_salinity . 13 convert_StoCl . 15 convert_StoR . 16 convert_T . 17 coriolis . 18 diffcoeff . 19 earth_surf . 21 gas_O2sat . 23 gas_satconc . 25 gas_schmidt . 27 gas_solubility . 28 gas_transfer . 31 gravity . 33 molvol . 34 molweight . 36 Oceans . 37 redfield . 38 ssd2rad . 39 sw_adtgrad . 40 sw_alpha . 41 sw_beta . 42 sw_comp . 43 sw_conserv . 44 sw_cp . 45 sw_dens . 47 sw_depth . 49 sw_enthalpy . 50 sw_entropy . 51 sw_gibbs . 52 sw_kappa . -

Calculation Exercises with Answers and Solutions
Atmospheric Chemistry and Physics Calculation Exercises Contents Exercise A, chapter 1 - 3 in Jacob …………………………………………………… 2 Exercise B, chapter 4, 6 in Jacob …………………………………………………… 6 Exercise C, chapter 7, 8 in Jacob, OH on aerosols and booklet by Heintzenberg … 11 Exercise D, chapter 9 in Jacob………………………………………………………. 16 Exercise E, chapter 10 in Jacob……………………………………………………… 20 Exercise F, chapter 11 - 13 in Jacob………………………………………………… 24 Answers and solutions …………………………………………………………………. 29 Note that approximately 40% of the written exam deals with calculations. The remainder is about understanding of the theory. Exercises marked with an asterisk (*) are for the most interested students. These exercises are more comprehensive and/or difficult than questions appearing in the written exam. 1 Atmospheric Chemistry and Physics – Exercise A, chap. 1 – 3 Recommended activity before exercise: Try to solve 1:1 – 1:5, 2:1 – 2:2 and 3:1 – 3:2. Summary: Concentration Example Advantage Number density No. molecules/m3, Useful for calculations of reaction kmol/m3 rates in the gas phase Partial pressure Useful measure on the amount of a substance that easily can be converted to mixing ratio Mixing ratio ppmv can mean e.g. Concentration relative to the mole/mole or partial concentration of air molecules. Very pressure/total pressure useful because air is compressible. Ideal gas law: PV = nRT Molar mass: M = m/n Density: ρ = m/V = PM/RT; (from the two equations above) Mixing ratio (vol): Cx = nx/na = Px/Pa ≠ mx/ma Number density: Cvol = nNav/V 26 -1 Avogadro’s -

Summary of a Program Review Held at Huntsville, Alabama October 19-21, 1982
Summary of a program review held at Huntsville, Alabama October 19-21, 1982 - TECH LIBRARY KAFEI, NM lllllllsllllllRlRllffllilrml OOSSE!?b NASA Conference Publication 2259 NASA/MSFCFY-82 Atmospheric Processes Research Review Compiled by Robert E. Turner George C. Marshall Space Flight Center Marshall Space Flight Center, Alabama Summary of a program review held at Huntsville, Alabama October 19-21, 1982 National Aeronautics and Space Administration Sclontlflc and Tochnlcal InformatIon Branch 1983 ACKNOWLEDGMENTS The productive inputs and comments from the participants and attendees in the Atmospheric Processes Research Review contributed very much to the success of the review. The opportunity provided for everyone to become better acquainted with the work of other investigators and to see how the research relates to the overall objective of NASA's Atmospheric Processes Research Program was an important aspect of the review. Appreciation is expressed to all those who participated in the review. The organizers trust that participation will provide each with a better frame of reference from which to proceed with the next year's research activities. ii PREFACE Each year NASA supports research in various disciplinary program areas. The coordination and exchange of information among those sponsored by NASA to conduct research studies are important elements of each program. The Office of Space Science and Applications and the Office of Aeronautics and Space Technology, via Announcements of Opportunity (AO), Application Notices (AN),etc., invites interested investigators throughout the country to communicate their research ideas within NASA and in institutions. The proposals in the Atmospheric Processes Research area selected and assigned to the NASA Marshall Space Flight Center's (MSFC's) Atmospheric Sciences Division for technical monitorship, together with the research efforts included in the FY-82 MSFC Research and Technology Operating Plan (RTOP1 I are the source of principal focus for the NASA/MSFC FY-82 Atmospheric Processes Research Review. -

Lecture 3. the Basic Properties of the Natural Atmosphere 1. Composition
Lecture 3. The basic properties of the natural atmosphere Objectives: 1. Composition of air. 2. Pressure. 3. Temperature. 4. Density. 5. Concentration. Mole. Mixing ratio. 6. Gas laws. 7. Dry air and moist air. Readings: Turco: p.11-27, 38-43, 366-367, 490-492; Brimblecombe: p. 1-5 1. Composition of air. The word atmosphere derives from the Greek atmo (vapor) and spherios (sphere). The Earth’s atmosphere is a mixture of gases that we call air. Air usually contains a number of small particles (atmospheric aerosols), clouds of condensed water, and ice cloud. NOTE : The atmosphere is a thin veil of gases; if our planet were the size of an apple, its atmosphere would be thick as the apple peel. Some 80% of the mass of the atmosphere is within 10 km of the surface of the Earth, which has a diameter of about 12,742 km. The Earth’s atmosphere as a mixture of gases is characterized by pressure, temperature, and density which vary with altitude (will be discussed in Lecture 4). The atmosphere below about 100 km is called Homosphere. This part of the atmosphere consists of uniform mixtures of gases as illustrated in Table 3.1. 1 Table 3.1. The composition of air. Gases Fraction of air Constant gases Nitrogen, N2 78.08% Oxygen, O2 20.95% Argon, Ar 0.93% Neon, Ne 0.0018% Helium, He 0.0005% Krypton, Kr 0.00011% Xenon, Xe 0.000009% Variable gases Water vapor, H2O 4.0% (maximum, in the tropics) 0.00001% (minimum, at the South Pole) Carbon dioxide, CO2 0.0365% (increasing ~0.4% per year) Methane, CH4 ~0.00018% (increases due to agriculture) Hydrogen, H2 ~0.00006% Nitrous oxide, N2O ~0.00003% Carbon monoxide, CO ~0.000009% Ozone, O3 ~0.000001% - 0.0004% Fluorocarbon 12, CF2Cl2 ~0.00000005% Other gases 1% Oxygen 21% Nitrogen 78% 2 • Some gases in Table 3.1 are called constant gases because the ratio of the number of molecules for each gas and the total number of molecules of air do not change substantially from time to time or place to place. -

The Stratopause Evolution During Different Types of Sudden Stratospheric Warming Event
Clim Dyn DOI 10.1007/s00382-014-2292-4 The stratopause evolution during different types of sudden stratospheric warming event Etienne Vignon · Daniel M. Mitchell Received: 18 February 2014 / Accepted: 5 August 2014 © The Author(s) 2014. This article is published with open access at Springerlink.com Abstract Recent work has shown that the vertical struc- Keywords Stratopause · Sudden stratospheric warming · ture of the Arctic polar vortex during different types of MERRA data · Polar vortex · sudden stratospheric warming (SSW) events can be very Middle atmospheric circulation distinctive. Specifically, SSWs can be classified into polar vortex displacement events or polar vortex splitting events. This paper aims to study the Arctic stratosphere during 1 Introduction such events, with a focus on the stratopause using the Mod- ern Era-Restrospective analysis for Research and Applica- The stratopause is characterised by a reversal of the atmos- tions reanalysis data set. The reanalysis dataset is compared pheric lapse rate at around 50 km (~1 hPa). While strato- against two independent satellite reconstructions for valida- spheric ozone heating is responsible for the stratopause pres- tion purposes. During vortex displacement events, the strat- ence at sunlit latitudes, westward gravity wave drag (and to opause temperature and pressure exhibit a wave-1 structure a lesser extent, stationary gravity wave drag) maintains the and are in quadrature whereas during vortex splitting events stratopause in the polar night jet (Hitchman et al. 1989). they exhibit a wave-2 structure. For both types of SSW the Indeed, the westward and stationary gravity wave (GW) temperature anomalies at the stratopause are shown to be breaking induces a mesospheric meridional flow toward the generated by ageostrophic vertical motions. -

Earth's Atmospheric Layers
Earth's atmospheric layers Earth's atmospheric layers Lesson plan (Polish) Lesson plan (English) Earth's atmospheric layers Source: licencja: CC 0, [online], dostępny w internecie: www.pixabay.pl. Link to the lesson Before you start you should know what the place of the atmosphere is in relation to the lithosphere, hydrosphere, biosphere and pedosphere; that the Earth's atmosphere is the part of the Earth and moves with it. You will learn explain the term „atmosphere”; name gases that form the air and their percentage share; name permanent and variable components of atmospheric air; name the layers of the atmosphere; discuss the role of the ozone layer; characterize the effects of the ozone hole and the greenhouse effect. Nagranie dostępne na portalu epodreczniki.pl nagranie abstraktu What layers is the atmosphere built of? In the Earth's atmosphere we distinguish 5 main layers characterized by specific features and 4 intermediate layers called pauses. The boundaries between them are conventional and change depending on the geographical latitude, terrain and season of the year. The closest one to the surface of the earth is the troposphere. Its thickness ranges from 7 km (in winter) to 10 km (in summer) above the poles, and 15‐18 km above the equator. The main feature that allows determining the boundary of the troposphere is the drop in the air temperature with an increase of about 0,6°C per 100 m. In the upper layer of the troposphere, the temperature reaches -55°C (above arctic regions) to -70°C (above equatorial regions). Above this layer there is a thin tropopause with the constant temperature, and above it there is the stratosphere extending up to a height of about 50 km, in which the air temperature rises to reach 0°C. -

Interactions Between Polymers and Nanoparticles : Formation of « Supermicellar » Hybrid Aggregates
1 Interactions between Polymers and Nanoparticles : Formation of « Supermicellar » Hybrid Aggregates J.-F. Berret@, K. Yokota* and M. Morvan, Complex Fluids Laboratory, UMR CNRS - Rhodia n°166, Cranbury Research Center Rhodia 259 Prospect Plains Road Cranbury NJ 08512 USA Abstract : When polyelectrolyte-neutral block copolymers are mixed in solutions to oppositely charged species (e.g. surfactant micelles, macromolecules, proteins etc…), there is the formation of stable “supermicellar” aggregates combining both components. The resulting colloidal complexes exhibit a core-shell structure and the mechanism yielding to their formation is electrostatic self-assembly. In this contribution, we report on the structural properties of “supermicellar” aggregates made from yttrium-based inorganic nanoparticles (radius 2 nm) and polyelectrolyte-neutral block copolymers in aqueous solutions. The yttrium hydroxyacetate particles were chosen as a model system for inorganic colloids, and also for their use in industrial applications as precursors for ceramic and opto-electronic materials. The copolymers placed under scrutiny are the water soluble and asymmetric poly(sodium acrylate)-b-poly(acrylamide) diblocks. Using static and dynamical light scattering experiments, we demonstrate the analogy between surfactant micelles and nanoparticles in the complexation phenomenon with oppositely charged polymers. We also determine the sizes and the aggregation numbers of the hybrid organic-inorganic 1 2 complexes. Several additional properties are discussed, such -

Methods of Oabservation at Sea Meteorological Soundings in The
WORLD METEOROLOGICAL ORGANIZATION WORLD METEOROLOGICAL ORGANIZATION TECHNICAL NOTE No. 2 TECHNICAL NOTE No. 60 METHODS OF OABSERVATION AT SEA METEOROLOGICAL SOUNDINGS IN THE PARTUPPER I – SEA SURFACEATMOSPHERE TEMPERATURE by W.W. KELLOGG WMO-No.WMO-No. 153. 26. TP. 738 Secretariat of the World Meteorological Organization – Geneva – Switzerland THE WMO The WOTld :Meteol'ological Organization (Wl\IO) is a specialized agency of the United Nations of which 125 States and Territories arc Members. It was created: to facilitate international co~operation in the establishment of networks of stations and centres to provide meteorological services and observationsI to promote the establishment and maintenance of systems for the rapid exchange of meteorological information, to promote standardization of meteorological observations and ensure the uniform publication of observations and statistics. to further the application of rneteol'ology to Rviatioll, shipping, agricultul"C1 and other human activities. to encourage research and training in meteorology. The machinery of the Organization consists of: The World Nleteorological Congress, the supreme body of the o.rganization, brings together the delegates of all Members once every four years to determine general policies for the fulfilment of the purposes of the Organization, to adopt Technical Regulations relating to international meteorological practice and to determine the WMO programme, The Executive Committee is composed of 21 dil'cetors of national meteorological services and meets at least once a yeae to conduct the activities of the Organization and to implement the decisions taken by its Members in Congress, to study and make recommendations Oll matters affecting international meteorology and the opel'ation of meteorological services. -
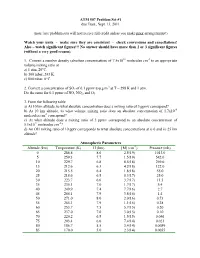
Problem Set #1 Due Tues., Sept
ATM 507 Problem Set #1 due Tues., Sept. 13, 2011 (note: late problem sets will not receive full credit unless you make prior arrangements!) Watch your units - make sure they are consistent - check conversions and cancellations! Also – watch significant figures!!! No answer should have more than 2 or 3 significant figures (without a very good reason). 1. Convert a number density (absolute concentration) of 7.5x1012 molecules cm-3 to an appropriate volume mixing ratio at a) 1 atm, 20°C. b) 100 mbar, 243 K. c) 800 mbar, 0°C. -3 2. Convert a concentration of SO2 of 0.1 ppmv to μg m at T = 298 K and 1 atm. Do the same for 0.1 ppmv of NO, NO2, and O3. 3. From the following table a) At 10 km altitude, to what absolute concentration does a mixing ratio of 3 ppmv correspond? b) At 10 km altitude, to what volume mixing ratio does an absolute concentration of 1.7x1010 molecules cm-3 correspond? c) At what altitude does a mixing ratio of 3 ppmv correspond to an absolute concentration of 5.1x1011 molecules cm-3? d) An OH mixing ratio of 10 pptv corresponds to what absolute concentrations at i) 0 and ii) 25 km altitude? Atmospheric Parameters Altitude (km) Temperature (K) H (km) [M] (cm-3) Pressure (mb) 0 288.8 8.6 2.5(19) 1013.0 5 259.3 7.7 1.5(19) 542.0 10 229.7 6.8 8.5(18) 269.0 15 212.6 6.3 4.2(18) 122.0 20 215.5 6.4 1.8(18) 55.0 25 218.6 6.5 8.3(17) 25.0 30 223.7 6.6 3.7(17) 11.5 35 235.1 7.0 1.7(17) 5.4 40 249.9 7.4 7.7(16) 2.7 45 266.1 7.9 3.8(16) 1.4 50 271.0 8.0 2.0(16) 0.73 55 265.3 7.9 1.1(16) 0.38 60 253.7 7.5 5.7(15) 0.20 65 237.0 7.0 3.0(15) 0.10 70 220.2 6.5 1.5(15) 0.046 75 203.4 6.0 7.4(14) 0.021 80 186.7 5.5 3.4(14) 0.0089 85 170.0 5.0 2.3(14) 0.0055 4.