4.0 Exploration/Production Activities and Associated Environmental Effects
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ecological Consequences Artificial Night Lighting
Rich Longcore ECOLOGY Advance praise for Ecological Consequences of Artificial Night Lighting E c Ecological Consequences “As a kid, I spent many a night under streetlamps looking for toads and bugs, or o l simply watching the bats. The two dozen experts who wrote this text still do. This o of isis aa definitive,definitive, readable,readable, comprehensivecomprehensive reviewreview ofof howhow artificialartificial nightnight lightinglighting affectsaffects g animals and plants. The reader learns about possible and definite effects of i animals and plants. The reader learns about possible and definite effects of c Artificial Night Lighting photopollution, illustrated with important examples of how to mitigate these effects a on species ranging from sea turtles to moths. Each section is introduced by a l delightful vignette that sends you rushing back to your own nighttime adventures, C be they chasing fireflies or grabbing frogs.” o n —JOHN M. MARZLUFF,, DenmanDenman ProfessorProfessor ofof SustainableSustainable ResourceResource Sciences,Sciences, s College of Forest Resources, University of Washington e q “This book is that rare phenomenon, one that provides us with a unique, relevant, and u seminal contribution to our knowledge, examining the physiological, behavioral, e n reproductive, community,community, and other ecological effectseffects of light pollution. It will c enhance our ability to mitigate this ominous envirenvironmentalonmental alteration thrthroughough mormoree e conscious and effective design of the built environment.” -

Portland Daily Press: March 16,1866
Maine State Library Digital Maine Portland Daily Press, 1866 Portland Daily Press 3-16-1866 Portland Daily Press: March 16,1866 Follow this and additional works at: https://digitalmaine.com/pdp_1866 Recommended Citation "Portland Daily Press: March 16,1866" (1866). Portland Daily Press, 1866. 63. https://digitalmaine.com/pdp_1866/63 This Text is brought to you for free and open access by the Portland Daily Press at Digital Maine. It has been accepted for inclusion in Portland Daily Press, 1866 by an authorized administrator of Digital Maine. For more information, please contact [email protected]. i r,.j» » _.. 8(fl .saK-i ‘•^'4fflSsfcFt'Ssasfc _..,_,_... 1 lo i»oial<Tri trfj 1*1 cd stci'WI ,iv, (Vot j,‘v., (<_ I;9Tofsqmi rood wedM blewUlfcv* 1 llll JV 1 VI. U vX llt..LI V7 j Lw b« «* *«5 | "^Mk» /\r% .?.* 5v CTTi^YJTT itnrTT t *. 7*m~~rvjf '.'" ... II ..... .- 1 lie 'Wliili ml 111 fj limiM** ......... .- __ >w.5. , FRIDAY PORTLAND, MARCH Terms $8 in advance. ■•■•;T- : MORNING, 16,1866. per annum, — ______ ._,_•.!.!__lx—xx_I ■_;_ THE PORTLAND DAILY PRESS U published Street, Business Notices. Miscellaneous. Miscellaneous. every day, (Sunday excepted,) at 82 Exchange Lost and Found. For Sale his A. Wants, and to Let* and Portland, N. Fostbr, Proprietor. | positions, his advice on some subjects Tebmo Dollars a In advance. ought not Eight year DAILY to be taken as sound or judicious. For A S. D. & H. W. Smith’s A WOUted. Sale. PRESS; voice comes at the Copartnership. 'ran, to us across the THE MAINE STATE PRESS. -

Men of Progress, 1898
Menf o Progress Biographical S ketches and Portraits OF Leaders i n Business and Professional Life INND A OF THE COMPILED U NDER THE SUPERVISION OF RICHARD H ERNDON EDITEDY B RICHARD B URTON BOSTON NEW E NGLAND MAGAZINE 1898 M5"3 Copvright, 1 897 uv RICHARD H ERNDON 7TKTrcq H lSTORICAC-1 • C. ALFRED M UDOE * SON, PRINTERS, BOSTON. MENF O PROGRESS. ALLEN, I saac Almarin, Jr., Architect, Hartford, a d escendant of Captain Ephraim Pease, who was born in Enfield street, Enfield, Connecticut, entertained General Washington at his house in May 22, 1859, son of Isaac Almarin and Harriet Enfield. His father's mother Mary (Pease) Allen Jane (Carrier) Allen. He is an only son; of his was also a descendant of Captain Ephraim Pease. four sisters, but one is now living — Elizabeth A letter from General Washington referring to the Ingraham (Allen) Burns, wife of Louis Burns of hospitality of Captain Pease, is still preserved by Pittsfield, Massachusetts. The other three sisters died while young. His father is a well-to-do farmer of Enfield, and his grandfather, Chauncey Allen, was an extensive farmer and dealer in leaf tobacco, who died at the age of eighty-nine, leaving a large property. Isaac Allen, brother of Chaun cey, moved from Enfield to Clarkson, Monroe county, New York, and became an extensive farmer there. At the age of eighteen he was a Colonel in the War of 1812. The genealogy of the family is traced back many generations in the Allen gene alogy, which has been published. On his mother's side he is descended from John Hancock, the signer of the Declaration of Independence. -

1880 Census: Volume 4. Report on the Agencies of Transportation In
ON :STEAM NA VIGArrION lN '.J.'Irn UNITED sr_rA 'l~ES. JJY SPECIAI..1 AGlt:.NT. i <65.'~ TABI"'E OF CONTENTS. Page. I .. BTTF.H OF TR A ~81\fITTAI.J ••• ~ - •• -- •••.•• - •• - •• - •• - • - •••• --- ••• - •••• -- •.•.••.••••••• - •••••• - ••• -- •••.•••••• - ••.• -- •••••••••• - • v C IIAPTBR. !.-HISTORY OF STEAM NA YI GA TION IN THE UNI'l'l~D STA TES. Tug EAHLY INVENTORS .•••••••••••••••••.••••••••••••••.••..••••••••••••••••••••••••••••••••••..•••••••••••••••••••••••••••• 1-4 11.ECOHDS OF CONSTRUCTION ..••••••••••.••••••••••••••••••...•••.••••••••••.••••••.•••••.•••••.••••••••.•••••••••....•••••••• 4,5 I~ec:1piti.1lation ......•••..........• , .......••.•......... -................•................••.•...•..••..•........•...... 5 LOCAL INTERESTS ••••. - ••••• - ••••••••••.•••••••.••. - •••..•• - ..•• - •••.••••.•.• -- ••••.•.••..••••.•••.•.• - •••••.•..• - •••••••.•• - • 5-7 Report of the Secretary of the 'rrensnry in 1838 .. ,. .................................................................... 5, 6· Report of the Secretary of tho 'l'reasnry in 1851. ....................................................................... • fi,7 INSPECTIONS OF STEAll! VESSELS ••••••..•••••••••••••• - ••••••••••. - •.••••••••••••••••••••••••.•.••••.••••••••••.•••..•••••••• 7 UNITED STATI~S AND l~ORBIGN TONNAGE ••••••••••• -- •••••••..•••..•••••••••••• -- • -- •••••• - ••••• ·--· .••• -· ••••••••••.•••••• - • 7,8 GRouP r.-NEw li::NGLANn sTA'l'Es •••••••••••••••••••.••••••••••••••••••.••••••••••••••••••••••••••••••.•••••.••••••••••••••• H-11 Building -

Asaa-Vsshstutber She Succeeded Bad Debt ..A-Ier:> Winds Islands, Glasgow: for New York
steamer State of Texas. Nlekertoa. Gatveston rl» ilj Ho.vo Ko*n. M«» bark Roaatta McNeil, Salled-ftteamer Vlndlrater. Rogers. Philadelphia; tek* AMUSEMENTS. THE MOUNT CARMEL TORNADO. OCEAN STEAMERS. West.C tl M A Co. Brown. San Franct-co: 27th, Wm II Diet*. Kndlcott. do. 5 L Simmons, Uandy. do. sllnry In June 1. bark Madura Stanton, Irom Cardiff, 3IK-Arriv«l, iMamir Wur, Crocker. Philadelphia; -learner Algiers. 11 awthorne. NewOrlcant.Bogort A Mor¬ port ,Br>, sehrs I II Borden. DATES OF DEPARTCRE KUOM NEW TORE FOR TOE gan. arrived May II. fir Sail rrancikoo HoiUS. KlliaOethnnri; Golden Rule. Wi|« Alt APPEAL »0* AID -DlBTnUCTIOS ASD DM* Steamer Now Orleans. Dearborn. New Or.aan*.Clark A flivtiu, June 31.Saileu, uaunir Irurac Bat tap), mi, N*w York; I'rhana. Allan, Itoboken, B M DuSietd, OILKOKE'* UARDLN. MONTHS or JLSfc AND Jl'LY. Baltimore. tiavnor. fort Johnson. Boa man. Arana. Sailed.Schrs J and titotiok im A o*ce TKWVWO WtailtBS SlMnwr. Ofkn. Steamer Saa Salvador. Nlcltanon. Savannah.George 11 a 1.1 f a x. Jhm 'JO.Hailed. Oar* Pactolu* (Br), Locxe, 8 Unrney, Gurney, Balls Seamau, A la evidently tba intention oI tbe managers .( this Tonga. London brlii Evviva iBri. Marvin. d>«. Stealman New York. resort to make it cool aa wail u musical. An town. Algeria 4 bowline Oraen Steamer Charleston. Lockwood. Charleston.J W tjuiutard Sailed Jlid. neauier Bermuda ( Br >. Angrove (Irom New 23d.S <lle 1. ach'i L A LvelL Borden. Philadelphia; popular J»ne 18T7. .V. St Johns. >P. Fanny Fern. Eaton: Louis Walsh, Comstock: American set on Moixi Cak>ieu HI., 21. -

A Maritime History of the Port of Whitby, 1700-1914
A MARITIME HISTORY OF THE PORT OF WHITBY, 1700-1914 - Submitted for the Degree of Doctor of Philosophy in the University of London STEPHANIE KAREN JONES UNIVERSITY COLLEGE LONDON 1982 2 A MARITIME HISTORY OF THE PORT OF WHITBY, 1700-1914 ABSTRACT This study attempts to contribute to the history of merchant shipping in a manner suggested by Ralph Davis, that 'the writing of substantial histories of the ports' was a neglected, but important, part of the subject of British maritime history. Rspects of the shipping industry of the port of Whitby fall into three broad categories: the ships of Whitby, built there and owned there; the trades in which these vessels were employed; and the port itself, its harbour facilities and maritime community. The origins of Whitby shipbuilding are seen in the context of the rise to prominence of the ports of the North East coast, and an attempt is made to quantify the shipping owned at Whitby before the beginning of statutory registration of vessels in 1786. A consideration of the decline of the building and owning of sailing ships at Whitby is followed by an analysis of the rise of steamshipping at the port. The nature of investment in shipping at Whitby is compared with features of shipowning at other English ports. An introductory survey of the employ- ment of Whitby-owned vessels, both sail and steam, precedes a study of Whitby ships in the coal trade, illustrated with examples of voyage accounts of Whitby colliers. The Northern Whale Fishery offered further opportunities for profit, and may be contrasted with the inshore and off - shore fishery from Whitby itself. -
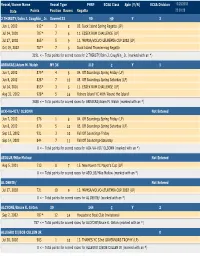
ECSA Class Spin (Y/N) ECSA Division 10/28/2002 Date Points Position Racers Regatta 22:00:28 2 THIRSTY/John J
Vessel/Owner Name Vessel Type PHRF ECSA Class Spin (Y/N) ECSA Division 10/28/2002 Date Points Position Racers Regatta 22:00:28 2 THIRSTY/John J. Coughlin_ Jr. Soverel 33 90 HP Y3 Jun 1, 2002912* 3 8 02. Duck Island Spring Regatta (LP) Jul 14, 2001707* 7 6 11. ESSEX RUM CHALLENGE (LP) Jul 27, 2002865* 5 9 13. MRMSA/VOLVO-LEUKEMIA CUP 2002 (LP) Oct 19, 2002707* 7 6 Duck Island Thundermug Regatta 3191 <-- Total points for scored races for 2 THIRSTY/John J. Coughlin_ Jr. (marked with an *) ABRAXAS/Adam M. Walsh NY 36 119 C Y1 Jun 7, 2002879* 4 8 04. Off Soundings Spring Friday (LP) Jun 8, 2002828* 7 10 05. Off Soundings Spring Saturday (LP) Jul 14, 2001853* 3 5 11. ESSEX RUM CHALLENGE (LP) Aug 31, 2002928* 5 16 Fishers Island YC 46th 'Round the Island' 3488 <-- Total points for scored races for ABRAXAS/Adam M. Walsh (marked with an *) ACK-VA-VIT/ OLSONN Not Entered Jun 7, 2002976 1 8 04. Off Soundings Spring Friday (LP) Jun 8, 2002879 5 10 05. Off Soundings Spring Saturday (LP) Sep 13, 2002931 3 10 Fall Off Soundings-Friday Sep 14, 2002844 7 11 Fall Off Soundings-Saturday 0 <-- Total points for scored races for ACK-VA-VIT/ OLSONN (marked with an *) AEOLUS/Mike Mollow Not Entered Aug 5, 2001713 8 7 15. New Haven YC Mayor's Cup (LP) 0 <-- Total points for scored races for AEOLUS/Mike Mollow (marked with an *) AL DENTE/ Not Entered Jul 27, 2002721 10 9 13. -

Organizing for Blaine. News From
RED BANK REGISTER VOLUME VII.. NO. 12. RED BANK, N. J., WEDNESDAY, SEPTEMBER 17,1884. $1.50 PER TEAR. ORGANIZING FOR BLAINE. PERSONAL. OBITUARY, NEWS FROM MIDDLETOWN, AN INTERESTING YAOHT RACE. IN AND OUT OP TOWN. FROM THE COPHTY 8KAT, William J. Crane, general agent for Danitl A. Walling. The HadtUna Take* Flnt Honey In Sbort and Imtereatlac lttmt trom Bed Bank and Vlclaltr. FORMATION OF THE BLAINE Chickering & Sons, was in town several Daniel A. Walling, of Keyport, a INTERESTING ITEMS FROM BE- Lut Satarda)r>* Regatta. A BUDCET OP INTIRUTINO AND LOOAN CLUB. days last week. young man 27 years of age, died at that YOND THE SHREWSBURY. The number of boats in the regatta on , A Butler and West campaign club NEWS FBOIS FltKEHOLP. Miss Lottie Udell, of Elizabeth, is on place on Saturday, September 6th. He BaptUm ml a Plenle-A Colored Elo. he South Shrewsbury last Saturday will be organized in Red Bank next Eighty-Six Hembors EnroU Their week. Hn»l« ••ring *atr Wack-VMSM* Nanus—OIan Booms to be Located ^Yiftit to her sister, lire. Goo. H. Wild, waa the oldest Bon of Jonathan B. and cutloQlst--Kvaporating Peachea and was far short of what was expected. Bvak* and a Watch Stal«B~a |Bk In tlcndrlckaon*' New Bulldlns- Kathrine Walling, and was a native cf Oilier Frail-Concert at Hlddletown There were but three to start in the race, The West End Hotel at Long Branch •war ~ *lt*t*riom». awaih - MM of Wallace street. -A Well-Patraulzcd Hall. *. Vl*oron« Compalgn to be Prose- George Mackey, of Keyport, was re- Keyport. -
![1868-03-21 [P ]](https://docslib.b-cdn.net/cover/1388/1868-03-21-p-5241388.webp)
1868-03-21 [P ]
of the I recalled I*oi*tlaii«t and At the annual of Hclig;loii)> In telligfenc©. the Eastern front Capitol. Vicinity. meeting tlio Portlaud Turn, SPECIAL. NOTLCE8. AGENTS KEW ADVEKTISEMliMTS. on the same on the 20th WANTEDl the time when, years ago, I stood rerein, inst., the following officers TVIK New AtberiiteneiiM tkii l»«f* The to the case to the marine were elected for the PTITgSB, Independent, retiring of, and, while locking up fig- ensuing year: Wm. Ross William Cullen and To tell an artlcio 1 spot, Bryant Male, Female, of unity. the un- as silent senti- Granville No Broker. Rev. Mr. Xyn., says Bishop Potter, by ures which genius had placed SPECIAL NOTICE COLUMN. Jr., Speaker; Batchelder, Secreta- Says cur Port Wine is very line, ana Eeem9 to meet No humbug Largo pr fits. Call and Morning, Msrolt 21, 1868. or adurcss with 20 cents, fjr Saturday necessary harshness of tone in his ad- nels around had a waking vision Charles H. John with general faver. investigate samples, and adopted the entrauce, California Wiue*—Peikins, Stern & Co. ry; Sawyer, Treasurer; C. directions, monition, and by the apparently studied insult of emancipation by the “war power”, which Moth Patches, &c.—B. C. reny. Dennis, Leader; John L. Shaw, Director; Surgeon-General Barnes STROUT & C«*.f First down with which was in the circumstances in the of SNTAMTAINM RN'T Charles E. The Says “all Wines submitted to tlio Gflaernment 93 Wasuiugtou St. room 2 gy~ Page to-day -Gqjgg conveyed j louud a place anti-slavery papers Small, Armorer. -

THE FIRST 40 YEARS TEEKAY the First 40 Years
BRIAN INGPEN TEEKAY THE FIRST 40 YEARS TEEKAY The First 40 Years Dedicated to the many loyal staff members of Teekay – past and present – who have helped the company earn a position at the forefront of the maritime industry. TEEKAY The First 40 Years Brian Ingpen Kattegat Limited PublishEd bY: Kattegat Limited 69 Pitts Bay Road Hamilton HM 08 Bermuda CONTENTS http://www.teekay.com/ First published 2013 Text © Brian Ingpen 2013 All rights reserved. No part of this book may be reproduced, stored in a retrieval system or transmitted in any form or by any means, without the prior written permission of the publisher. EdiTor: Douglas van der Horst Foreword vii ProjEcT mAnAgEr: Douglas van der Horst Chairman’s Perspective viii dEsign And layouT: The Nimble Mouse My Brother and Teekay xi dusTjAcKET dEsign: The Nimble Mouse Proof-rEAdEr: Tessa Kennedy Author’s Preface xiii indExEr: Ethleen Lastovica Hirt & Carter Cape (Pty) Ltd rEProducTion: 1 A Danish Farm Boy 17 PrinTing And binding: Tien Wah Press (Pte) Ltd, Singapore 2 American Dawn 29 3 A Cedar Has Fallen 59 ISBN 978-0-620-56155-6 4 Boom Years 93 5 Wider Horizons 153 In 2012 Teekay commissioned the well- known artist Ian Marshall to paint the Afterword 185 watercolours and sketches that appear at Appendix 1: Teekay’s Departmental Structure 187 various places throughout the text. These evocative works have added greatly to the Appendix 2: Recent Changes to Teekay’s Structures and Procedures 190 illustrative impact of the book. Appendix 3: Fleet list 192 Appendix 4: Chronology 199 Appendix 5: Ship Types and Glossary 202 Appendix 6: Typical Areas of Operation 204 Index 206 Foreword eadership is a service, it is not there to be with its most senior executives – one-to-one as reliability, integrity and teamwork are not L served. -
The Only Recent Reports of This Species in Conception Bay Consist of One To
Labrador‐Island Transmission Link Environmental Impact Statement Chapter 10 Existing Biophysical Environment The only recent reports of this species in Conception Bay consist of one to two males and a Barrow’s Goldeneye X Common Goldeneye hybrid male that have wintered for over ten years in Spaniard’s Bay, 22 km northwest of the proposed shoreline electrode site (Mactavish 2010, pers. comm.; Schmelzer 2006). Piping Plover 5 Piping Plover, melodus subspecies, a shorebird, is listed on Schedule 1 of SARA as ‘Endangered’ and on the NLESA as ‘Endangered’ (May 2001) (Table 10.5.10‐8). This species was last assessed by COSEWIC in May 2001 (COSEWIC 2010c, internet site). Reasons for its designation include a small number of individuals that are breeding in Canada, and a decreasing quality, loss and destruction of nesting habitat. Predation, habitat degradation by ATV use and other disturbances are interfering with reproductive success. Strong conservation 10 initiatives have failed to result in any substantial increase in numbers of breeding pairs (COSEWIC 2010c, internet site). Piping Plover nest on some of the sandy beaches on Newfoundland’s west and south coasts, the closest to the proposed submarine cable crossing corridor being 175 km to the south‐west at Shallow Bay in Gros Morne National Park (Sikumiut 2010b). The closest nesting location to the proposed Dowden’s Point shoreline 15 electrode site is 250 km to the south‐west at St. Pierre and Miquelon (CWS 2004c, internet site). Piping Plover may occur within the proposed corridor during pre‐ or post‐breeding movements. Eskimo Curlew The Eskimo Curlew is a species of shorebird that is listed on Schedule 1 of SARA and NLESA as ‘Endangered’ (Table 10.5.10‐8). -

The Poetry of John Tyndall COMPARATIVE LITERATURE and CULTURE
The Poetry of John Tyndall COMPARATIVE LITERATURE AND CULTURE Series Editors TIMOTHY MATHEWS AND FLORIAN MUSSGNUG Comparative Literature and Culture explores new creative and critical perspectives on literature, art and culture. Contributions offer a comparative, cross-cultural and interdisciplinary focus, showcasing exploratory research in literary and cultural theory and history, material and visual cultures, and reception studies. The series is also interested in language-based research, particularly the changing role of national and minority languages and cultures, and includes within its publications the annual proceedings of the ‘Hermes Consortium for Literary and Cultural Studies’. Timothy Mathews is Emeritus Professor of French and Comparative Criticism, UCL. Florian Mussgnug is Reader in Italian and Comparative Literature, UCL. EthicsTheCanada Poetry and in the FrameAesthetics of ofJohnCopyright, Translation CollectionsTyndall and the Image of Canada, 1895– 1924 Exploring the Work of Atxaga, Kundera and Semprún HarrietPhilip J. Hatfield Hulme Edited by Roland Jackson, Nicola Jackson and Daniel Brown 00-UCL_ETHICS&AESTHETICS_i-278.indd9781787353008_Canada-in-the-Frame_pi-208.indd 3 3 11-Jun-1819/10/2018 4:56:18 09:50PM First published in 2020 by UCL Press University College London Gower Street London WC1E 6BT Available to download free: www.uclpress.co.uk Collection © Editors, 2020 Text © Contributors, 2020 The authors have asserted their rights under the Copyright, Designs and Patents Act 1988 to be identified as the authors of this work. A CIP catalogue record for this book is available from The British Library. This book is published under a Creative Commons 4.0 International licence (CC BY 4.0). This licence allows you to share, copy, distribute and transmit the work; to adapt the work and to make commercial use of the work providing attribution is made to the authors (but not in any way that suggests that they endorse you or your use of the work).