Host Use by Generalist and Specialist Brood-Parasitic Cowbirds at Population and Individual Levels
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
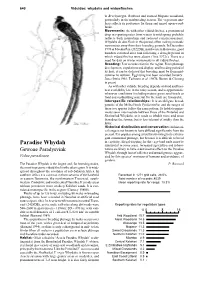
Paradise Whydah Substantial in Some Areas
640 Viduidae: whydahs and widowfinches in Brachystegia, Baikiaea and stunted Mopane woodland, particularly in the nonbreeding season. The vegetation ana- lysis reflects its preference for thorn and mixed open wood- lands. Movements: As with other viduid finches, a pronounced drop in reporting rates from winter to mid-spring probably reflects both nomadism and reduced conspicuousness. Whydahs do also flock in this period, often making nomadic movements away from their breeding grounds. In December 1970 at Mwaku Pan (2022DB), northwestern Botswana, good numbers returned after rain following a drought period in which viduid finches were absent (Tree 1972c). There is a need for data on winter movements in all viduid finches. Breeding: Few records exist for the region. From plumage development, copulations and display, and breeding period of its host, it can be deduced that breeding must be from mid- summer to autumn. Egglaying has been recorded January– June (Irwin 1981; Tarboton et al. 1987b; Brown & Clinning in press). As with other viduids, breeding depends on food and host- nest availability late in the rainy season, and is opportunistic whenever conditions (including mature green seed-heads as food and nestbuilding material for the host) are favourable. Interspecific relationships: It is an obligate brood- parasite of the Melba Finch Pytilia melba, and the ranges of these two species follow the same pattern. Its habitat require- ments seem intermediate between those of the Pintailed and Shafttailed Whydahs, as it tends to inhabit more arid areas than does the former, but is less tolerant of aridity than the latter. Historical distribution and conservation: Its histori- cal range is not known to have differed significantly from the present. -

Manyoni Private Game Reserve (Previously Zululand Rhino Reserve)
Manyoni Private Game Reserve (Previously Zululand Rhino Reserve) Gorgeous Bushshrike by Adam Riley BIRD LIST Prepared by Adam Riley [email protected] • www.rockjumperbirding. -

Egg Laying Behavior of Common Cuckoos (Cuculus Canorus): Data Based on Field Video-Recordings
ZOOLOGICAL RESEARCH Egg laying behavior of common cuckoos (Cuculus canorus): Data based on field video-recordings DEAR EDITOR, terms of time, some parasites, such as common cuckoos, are The egg laying behavior of brood parasites is at the heart of known to lay their eggs in the afternoon or evening (Nakamura studies on host co-evolution. Therefore, research on egg et al., 2005; Wyllie, 1981). As hosts lay eggs and stay close to laying behavior can improve our understanding of brood the nest in the morning, but rarely in the afternoon, afternoon parasitism and associated processes. Over a seven year parasitism reduces the risk of detection by the host and the study period, we monitored 455 oriental reed warbler possibility of parasitic egg rejection (Davies & de L. Brooke, (Acrocephalus orientalis) nests during the egg laying period, 1988). However, some parasites such as cowbirds (Molothrus 250 of which were parasitized by common cuckoos (Cuculus spp.) complete egg laying before sunrise (Gloag et al., 2013; canorus). We collected 53 clear videos of common cuckoo Sealy, 1992). The entire process of egg laying by a brood parasitism, analyzed all recorded parasitic behavior in detail, parasite is usually short (less than 10 s) (Davies & de L. and summarized the process of brood parasitism. Brooke, 1988; Ellisson et al., 2020; Moksnes et al., 2000; Furthermore, based on analyses of the field video recordings, Scardamaglia et al., 2017; Soler & Soler, 2000); despite this, we propose a new explanation for egg removal behavior, in addition to egg laying, brood parasites also display other namely the delivery hypothesis, i.e., egg pecking and biting by complex behaviors. -

1 Convergent Evolution of Reduced Eggshell Conductance in Avian
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Royal Holloway - Pure Convergent evolution of reduced eggshell conductance in avian brood parasites Stephanie C. McClelland1*, Gabriel A. Jamie2,3, Katy S. Waters1, Lara Caldas1, Claire N. Spottiswoode2,3 and Steven J. Portugal1 1School of Biological Sciences, Royal Holloway University of London, Egham, Surrey, TW20 0EX, UK. 2Department of Zoology, University of Cambridge, Downing Street, University of 10 Cambridge, Cambridge CB2 3EJ, UK. 3FitzPatrick Institute of African Ornithology, DST-NRF Centre of Excellence, University of Cape Town, Rondebosch 7701, Cape Town, South Africa. Subject Areas: evolution, parasitism, physiology Keywords: Cuculidae, eggs, gas exchange, Indicatoridae, Viduidae Author for Correspondence: Stephanie C. McClelland, email: [email protected] 20 1 Abstract Brood parasitism has evolved independently in several bird lineages, yet these species 30 share many similar physiological traits that optimise this breeding strategy, such as shorter incubation periods and thicker eggshells. Eggshell structure is important for embryonic development as it controls the flux of metabolic gases, such as O2, CO2 and H2O, into and out of the egg; in particular, water vapour conductance (GH2O) is an essential process for optimal development of the embryo. Previous work has shown that common cuckoos (Cuculus canorus) have a lower than expected eggshell GH2O compared to their hosts. Here we sought to test whether this is a trait found in other independently-evolved avian brood parasites, and therefore reflects a general adaptation to a parasitic lifestyle. We analysed GH2O for seven species of brood parasites from four unique lineages as well as for their hosts, and combined this with species 40 from the literature. -

Southern Tanzania: Endemic Birds & Spectacular Mammals
SOUTHERN TANZANIA: ENDEMIC BIRDS & SPECTACULAR MAMMALS SEPTEMBER 18–OCTOBER 6, 2018 Tanzanian Red-billed Hornbill © Kevin J. Zimmer LEADERS: KEVIN ZIMMER & ANTHONY RAFAEL LIST COMPILED BY: KEVIN ZIMMER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM SOUTHERN TANZANIA: ENDEMIC BIRDS & SPECTACULAR MAMMALS September 18–October 6, 2018 By Kevin Zimmer After meeting in Dar es Salaam, we kicked off our inaugural Southern Tanzania tour by taking a small charter flight to Ruaha National Park, at 7,800 square miles, the largest national park in all of east Africa. The scenery from the air was spectacular, particularly on our approach to the park’s airstrip. Our tour was deliberately timed to coincide with the dry season, a time when many of the trees have dropped their leaves, heightening visibility and leaving the landscapes starkly beautiful. This is also a time when the Great Ruaha River and its many smaller tributaries dwindle to shallow, often intermittent “sand rivers,” which, nonetheless, provide natural game corridors and concentration points for birds during a time in which water is at a premium. Bateleur, Ruaha National Park, Sept 2018 (© Kevin J. Zimmer) After touching down at the airstrip, we disembarked to find our trusty drivers, Geitan Ndunguru and Roger Mwengi, each of them longtime friends from our Northern Tanzania tours, waiting for us with their safari vehicles ready for action. The first order of business was to head to the lodge for lunch, but a large, mixed-species coven of Victor Emanuel Nature Tours 2 Southern Tanzania, 2018 vultures could not be ignored, particularly once we discovered the reason for the assemblage—a dead Hippo, no doubt taken down the previous night as it attempted to cross from one river to another, and, the sated Lion that had been gorging itself ever since. -

Assessment of Farming Systems and Cover Configuration Options That Enhance Natural Regulation of Herbivorous Arthropod Abundance in Maize-Fields
Assessment of farming systems and cover configuration options that enhance natural regulation of herbivorous arthropod abundance in maize-fields by Nickson E. Otieno Dissertation presented for the degree of Doctor of Philosophy (Conservation Ecology) at Stellenbosch University Conservation Ecology and Entomology, Faculty of AgriSciences Supervisor: Dr. Shayne M. Jacobs Co-supervisor: Dr. James S. Pryke December 2018 i Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated) that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. Date: December 2018 Copyright © 2018 Stellenbosch University All rights reserved ii Stellenbosch University https://scholar.sun.ac.za Dissertation format This dissertation is presented as a compilation of 6 chapters. Each chapter is introduced separately and is written, including reference citing and list of bibliography, according to the style of the journal Agriculture, Ecosystems and Environment. The following chapters have already been submitted for publication in journals Chapter 3: Otieno NE, Pryke, JS, Jacobs, SM. The top-down suppression of arthropod herbivory in inter-cropped maize and organic farms: evidence from δ13C and δ15N stable isotope analyses. Journal of Agronomy for Sustainable Development. Chapter 4: Otieno NE, Jacobs, SM, Pryke, JS. Influence of farm structural complexity features and farming systems on insectivorous birds’ contribution to arthropod herbivore regulation in maize fields. -

The Brown-Headed Cowbird: a Model Species for Testing Novel Research 9 Questions in Animal Ecology, Evolution, and Behavior
The Brown-Headed Cowbird: A Model Species for Testing Novel Research 9 Questions in Animal Ecology, Evolution, and Behavior Brian D. Peer, James W. Rivers, Loren Merrill, Scott K. Robinson, and Stephen I. Rothstein Abstract Although the brown-headed cowbird (Molothrus ater) is the most intensively studied brood parasite in the world, much of the research on cowbirds has focused on the negative effects of parasitism. Here, we argue that negative attitudes toward the cowbird have overshadowed opportunities this species provides for advancing our understanding of social behavior, physiology, evolution, and ecology and conservation of birds. Cowbirds are widely distributed, amenable to captive study, and easy to study in areas where they are abundant. Cowbird nestlings must communicate to unrelated host parents, but unlike some parasitic nestlings, they have no specialized adaptations for doing so. In some areas they often share nests with relatives, which may influence the degree of virulence host experience. The generalist strategy of the cowbird can be used to answer questions about the impact of high reproductive output on female cowbirds, maternal allocation of resources into eggs, and the consequences of exposure to a range of pathogens while visiting host nests. Cowbirds and their hosts provide a contrast to cuckoo-host systems because they are at an earlier stage of B. D. Peer (*) Department of Biological Sciences, Western Illinois University, Moline, IL, USA e-mail: [email protected] J. W. Rivers Department of Forest Ecosystems and Society, Oregon State University, Corvallis, OR, USA L. Merrill Illinois Natural History Survey, Prairie Research Institute, University of Illinois, Champaign, IL, USA S. -

Uganda and Rwanda: Shoebill Experience, Nyungwe’S Albertine Rift and Great Apes
MEGAFARI 2: Uganda and Rwanda: Shoebill experience, Nyungwe’s Albertine Rift and Great Apes 27 July – 7 August 2010 (12 days), Leader: Keith Barnes, Custom trip Photos by Keith Barnes. All photos taken on this trip. The spectacular Green-breasted Pitta was the star of the show in Uganda. We found only the sixth-ever nest of this species and spent a few hours with it gathering valuable information on the breeding biology of this species. Here a male droops his wings and displays to a female on the ground. Introduction This was the first leg of our second Megafari of 2010 – a true trip of a lifetime for most of the participants. The main aims of the Uganda and Rwanda leg was to see a Shoebill stalking in deep Papyrus swamps, attempt to see the most unlikely scarce Central African denizen, Green-breasted Pitta, score a gamut of rainforest birds in both the lowlands of Kibale NP and then also the impressive montane forests of the incredible Nyungwe NP, and to see primates, and of course, the irrepressible great apes, Chimpanzee and Mountain Gorilla. Fortunately, we achieved all these aims, netting 346 bird species on this 12-day leg of the trip, of which only 9 were spent birding, as well as accumulating an incredible 652 bird species and 60 mammals in just over four-weeks of the Megafari. The Megafari was a boon for spectacular birds and we saw 32 species of bird of prey, 8 species of turaco, 7 species of kingfisher, 8 species of bee-eater, 9 species of hornbill, and 28 species of sunbird. -

Goodvin 2018.Pdf
Goodvin 2 INTRODUCTION Brood-parasitism is an unequal relationship between one member (the parasite) and the other (the host), which likely results in an evolutionary arms race. An evolutionary arms race is the ongoing competition of co-evolving genes in both members of the relationship, which evolve to counteract the others’ genes, resulting in a constant race to be able to defend or to overcome the others’ defenses. The cuckoos (Cuculidae) are another type of avian brood-parasite and have been studied extensively (Poiani and Elgar, 1992; Hughes, 1996; Hughes, 1997). The waxbills (Estrildidae) are a family of small passerines existing in the Afrotropical, Oriental, and Australasian regions, as well as some tropical Pacific islands. Within the Estrildidae, two genera, Estrilda and Amandava, include species which construct unique nest structures previously referred to as “cock’s nests” (Chapin, 1954; Goodwin, 1982). These structures are open-cupped and built on top of the existing, covered nest structure; their function is unknown (Payne and Bonan, 2018). According to previous observations, only the male spends time in this structure and will rub old droppings into it (Payne and Bonan, 2018). Some possible explanations for the creation of these false nests are that it is a decoy for predators (Galligan and Kleindorfer, 2008), a sexually selected for trait, or that it may be a decoy for brood-parasites, the last of which this study will focus on. The Estrildidae are commonly parasitized by the whydahs and indigobirds (Viduidae), which are small, brood-parasitic passerines living in sub-Saharan Africa. They have evolved to specialize on waxbills of the genera Estrilda and Amandava as their hosts (Payne and Bonan, 2018). -

Nest Predation by Brown-Headed Cowbirds (Molothrus Ater)
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 3-28-2018 2:00 PM Nest Predation by brown-headed cowbirds (Molothrus ater) David C. Swan The University of Western Ontario Supervisor Zanette, Liana Y. The University of Western Ontario Graduate Program in Biology A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © David C. Swan 2018 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Biology Commons Recommended Citation Swan, David C., "Nest Predation by brown-headed cowbirds (Molothrus ater)" (2018). Electronic Thesis and Dissertation Repository. 5274. https://ir.lib.uwo.ca/etd/5274 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. Abstract The reproductive success of parasites is entirely dependent on their ability to encounter suitable hosts. Obligate brood parasitic birds may increase host encounter rate, and consequently their reproductive output, if they cause unsuitable late-stage host nests to fail thereby stimulating the host to create another nest that they can parasitize. I tested key predictions of this ‘farming’ hypothesis for the brown-headed cowbird (Molothrus ater). I found evidence that cowbird attacks are not uncommon, a basic requirement of the hypothesis. Furthermore, I found multiple lines of evidence that cowbird attacks are not indiscriminate, but directed at non-parasitized nests and at those at a developmental stage too late to be suitable for parasitism. -

Visual Trickery in Avian Brood Parasites
CHAPTER 6 Visual trickery in avian brood parasites N aomi E . L angmore and C laire N . S pottiswoode 6. 1 Introduction into rearing its young. Thus we generally see the most advanced parasite recognition systems Visual mimicry is a strategy deployed by a wide amongst hosts of evicting parasites, which in turn range of taxa, from spiders that mimic the ants they select for highly accurate visual mimicry by para- hunt ( Nelson and Jackson 2006 ), to cross-dressing sites to evade host detection. males in bluegill sunfi sh ( Dominey 1980 ), and However, host defenses are not the only selective harmless snakes that mimic the vibrant colors of agent responsible for the evolution of visual mimicry venomous snakes ( Harper and Pfenning 2008 ) (see in brood parasites. Mimicry can also arise to facilitate Chapter 11 ). Although visual mimicry is wide- exploitation of parent–offspring channels of commu- spread amongst invertebrates, and to a lesser extent nication, ensuring that the parasite chick is able to fi sh, amphibians, and reptiles ( Ruxton et al. 2004 ), elicit adequate care from its foster parents (parasite it is generally a rare phenomenon among birds (but “tuning,” Davies 2011 ). Mimicry for this purpose is see e.g., Dumbacher and Fleischer 2001 ). However, equally likely to arise in evicting and non-evicting the avian brood parasites provide a notable excep- parasites, and was termed sequential evolution mim- tion. Several recent fi eld studies, combined with icry by Grim ( 2005 ), to distinguish it from mimicry new techniques for quantifying mimicry and mod- that arises through a coevolutionary arms race. -

Checklist of the Birds of Boni-Dodori
CHECKLIST OF THE BIRDS OF BONI - DODORI CHECKLIST OF THE BIRDS OF BONI - DODORI IBA Cover: Red-headed Weaver, Juba race Top right: Yellowbill, migrant from the south Top left: Common Cuckoo, migrant from the north Below: Senegal Plover ALL PHOTOS BY JOHN MUSINA CHECKLIST OF THE BIRDS OF BONI - DODORI IBA CHECKLIST OF THE BIRDS OF BONI - DODORI The Boni-Dodori Forest System The Boni-Dodori forest system is in the easternmost corner of Kenya, bordering Somalia and the Indian Ocean. It comprises Boni and Dodori National Reserves, Boni- Lungi and Boni-Ijara forests (which at the time of publication were understood to have recently been gazetted as Forest Reserves) and the Aweer Community Conservancy, proposed by the indigenous Aweer (Boni) people and the Northern Rangelands Trust. The Boni-Dodori area was designated Kenya’s 63rd Important Bird and Biodiversity Area (IBA) by Nature Kenya and BirdLife International in 2014. It forms part of the East African coastal forests biodiversity hotspot, an area known for globally significant levels of species richness and one of Africa’s centers of endemism. At the time of going to press, the area was under the control of the Kenya Defence Forces with restricted movement of the public. It is hoped that security will soon be restored and this remarkable landscape will be open to visitors again. This Checklist will be the first guide for visitors. The Landscape The Boni-Dodori forest system is a vast mosaic of east African coastal forest and thicket, seasonally flooded grassland and palm savanna, scattered wetlands and a strip of Acacia woodland.