Visual Trickery in Avian Brood Parasites
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Birds of Gag Island, Western Papuan Islands, Indonesia
DOI: 10.18195/issn.0312-3162.23(2).2006.115-132 [<ecords o{ the rVcstCrJJ Australian Museum 23: 115~ n2 (2006). The birds of Gag Island, Western Papuan islands, Indonesia R.E. Johnstone Western Australian Museum, Locked Bag 49, Welsh pool DC Western Australia 6'1S6 Abstract This report is based mainly on data gathered during a biological survey of C;ag Island by a joint Western Australian Museum, Museum Zoologicum Bogoriense and Herbarium Bogoriense expedition in July 1'197. A total of 70 species of bird have been recorded for Gag Island and a number of these represent new island and/or Raja Ampat Archipelago records. Relative abundance, status, local distribution and habitat preferences found for each species arc described, extralimital range is outlined and notes on taxonomv are also given. No endemic birds were recorded for Gag Island but a number of species show significant morphological variation from other island forms and may prove to be distinct taxonomically. INTRODUCTION undergo geographic variation for taxonon,ic, Gag Island (0025'S, 129 U 53'E) is one of the Western morphological and genetic studies. The Papuan or Raja Ampat Islands, lying just off the annotated checklist provided covers every Vogelkop of Irian Jaya, between New Guinea and species recorded, both historically and during Halmahera, Indonesia. These islands include (from this survey. north to south) Sayang, Kawe, Waigeo, Gebe, Gag, In the annotated list I summarise for each species Gam, Batanta, Salawati, Kofiau, Misool and a its relative abundance (whether it is very common, number of small islands (Figure 1). Gag Island is common, moderately common, uncommon, scarce separated from its nearest neighbours Gebe Island or rare), whether it feeds alone or in groups, status to the north~west, and Batangpele Island to the (a judgement on whether it is a vagrant, visitor or north-east, by about 40 km of relatively deep sea. -

Jacobin Cuckoo of Either the Cape Bulbul P
556 Cuculidae: cuckoos and coucals months later. In Botswana, departure time was correlated with total rainfall during the summer, birds being recorded two months later at the end of a wet year than during drought years (Herremans 1994d). Differences between the subspecies in timing of occurrence and different ranges within the region, if any, have not been unravelled. Breeding: It is a brood parasite whose prime hosts are Pycnonotus bulbuls, the Sombre Bulbul Andropadus impor- tunus, and the Fiscal Shrike Lanius collaris (Rowan 1983; Maclean 1993b). Egglaying has been recorded in the region October–April, with a peak November–January (Dean 1971; Irwin 1981; Rowan 1983; Tarboton et al. 1987b; Skinner 1996a; Brown & Clinning in press). The atlas data suggest breeding to be a month later than recorded in the literature, but this results from a bias towards the recording of recently fledged young. Interspecific relationships: Because the distribution map represents both breeding and nonbreeding visitors, it is not straightforward to relate it to the distributions of host species. Breeding was, however, reported from all Zones and it can be deduced that it must use Blackeyed Pycnonotus barbatus and Redeyed P. nigricans Bulbuls extensively as hosts, but there is no distributional evidence for exclusive use Jacobin Cuckoo of either the Cape Bulbul P. capensis or the Sombre Bulbul. Bontnuwejaarsvoël The bulbuls, however, all have parts of their range where the cuckoo does not occur, but least so for the Blackeyed Bulbul. Clamator jacobinus Historical distribution and conservation: It was once quite common in the Cape Peninsula (3418A) but is now only The Jacobin Cuckoo is widespread in the Afrotropical a rare visitor to the southwestern Cape Province (Rowan 1983; savannas, both north and south of the equator (Fry et al. -

REGUA Bird List July 2020.Xlsx
Birds of REGUA/Aves da REGUA Updated July 2020. The taxonomy and nomenclature follows the Comitê Brasileiro de Registros Ornitológicos (CBRO), Annotated checklist of the birds of Brazil by the Brazilian Ornithological Records Committee, updated June 2015 - based on the checklist of the South American Classification Committee (SACC). Atualizado julho de 2020. A taxonomia e nomenclatura seguem o Comitê Brasileiro de Registros Ornitológicos (CBRO), Lista anotada das aves do Brasil pelo Comitê Brasileiro de Registros Ornitológicos, atualizada em junho de 2015 - fundamentada na lista do Comitê de Classificação da América do Sul (SACC). -

The Birds of Babar, Romang, Sermata, Leti and Kisar, Maluku, Indonesia
Colin R. Trainor & Philippe Verbelen 272 Bull. B.O.C. 2013 133(4) New distributional records from forgoten Banda Sea islands: the birds of Babar, Romang, Sermata, Leti and Kisar, Maluku, Indonesia by Colin R. Trainor & Philippe Verbelen Received 5 July 2011; fnal revision accepted 10 September 2013 Summary.—Many of the Banda Sea islands, including Babar, Romang, Sermata and Leti, were last surveyed more than 100 years ago. In October–November 2010, birds were surveyed on Romang (14 days), Sermata (eight days), Leti (fve days) and Kisar (seven days), and on Babar in August 2009 (ten days) and August 2011 (11 days). Limited unpublished observations from Damar, Moa, Masela (of Babar) and Nyata (of Romang) are also included here. A total of 128 bird species was recorded (85 resident landbirds), with 104 new island records, among them fve, 12, 20, four and three additional resident landbirds for Babar, Romang, Sermata, Leti and Kisar, respectively. The high proportion of newly recorded and apparently overlooked resident landbirds on Sermata is puzzling but partly relates to limited historical collecting. Signifcant records include Ruddy-breasted Crake Porzana fusca (Romang), Red-legged Crake Rallina fasciata (Sermata), Bonelli’s Eagle Aquila fasciata renschi (Romang), Elegant Pita Pita elegans vigorsii (Babar, Romang, Sermata), Timor Stubtail Urosphena subulata (Babar, Romang), the frst sound-recordings of Kai Cicadabird Coracina dispar (Babar?, Romang) and endemic subspecies of Southern Boobook Ninox boobook cinnamomina (Babar) and N. b. moae (Romang, Sermata?). The frst ecological notes were collected for Green Oriole Oriolus favocinctus migrator on Romang, the lowland-dwelling Snowy-browed Flycatcher Ficedula hyperythra audacis on Babar, the endemic subspecies of Yellow- throated (Banda) Whistler Pachycephala macrorhyncha par on Romang, and Grey Friarbird Philemon kisserensis on Kisar and Leti. -

N a T U R E P R O G R a M S F I E L D T R I
EASTERN LONG ISLAND AUDUBON SOCIETY – From the Barrens to the Bays Formerly Moriches Bay Audubon, established 1967 November/December 2008 —Vol. XXXVIII No. 6 NATURE PROGRAMS J a Monday, November 3rd m e s NY'S SECOND BREEDING BIRD ATLAS G a l l BY KIMBERLEY CORWIN e t t o NewYork’s second Breeding Bird Atlas is imminent. The book documents changes in our bird distributions over the past 20 years. Some species have increased greatly while others have declined alarmingly. A few species are new breeders in the state while at least one has all but disappeared. Kimberley Corwin, who coordinated the Atlas project and co-edited the Atlas pub - lication, will show distribution maps and share some of the stories with us. Get a sneak peak into the book that NewYork's birders have been waiting years to see! The first feeding from A RedTale Kim is the Co-Editor of the Breeding Bird Atlas publication. She served as the Monday, December 1st James Galletto has been photographing Project Coordinator of the project from JAMES GALLETTO - A RED TALE nature for ten years and is known world its start in 1999. Kim holds a Master's de - wide for his action and behavior photog - James Galletto will give a program called gree in Ecology and Evolutionary Biology raphy. His images have graced the cover of A RedTale an intimate view inside the from the University at Albany. In her spare Natures Best Magazine and have hung on life of a Red-tail Hawk Family. We will fol - time, Kim enjoys hiking and birding. -

P0483-P0485.Pdf
SHORT COMMUNICATIONS The Condor 86:483-485 was seen in any of the unparasitizednests, including two 0 The Cooper Ornithological Society 1984 that were checked on the day prior to hatching. No damage was seen on the two infertile host eggsin DAMAGED WESTERN FLYCATCHER parasitized nests (Table 1). One of these eggsremained in EGGS IN NESTS CONTAINING the nest after the cowbird chick had fledged, still without sign of damage. BROWN-HEADED COWBIRD The severity of damage was correlated with the age of CHICKS the cowbird chick (Table 1). Small nicks were present on an egg in a nest with a cowbird as young as two days old, but extensivedamage was not seenuntil the chick was five PATRICIA M. DOLAN days old. In no case did flycatcher young remain in the nest AND with the cowbird chick for as long as two days after host PHILIP L. WRIGHT hatching. Most of the host eggsdisappeared within a day or two after they showed signs of hatching. We assumed them to have producedyoung that subsequentlydied and The young of many brood parasitesbehave in ways that were removed by the parents. harm the host young with which they share a nest. By ejecting or injuring their nestmates, they reduce compe- DISCUSSION tition for parental care. To our knowledge,no specificanti- We can offer severalpossible explanations for the damage host behavior has been reported for chicks of the Brown- observed. headed Cowbird (Molothrus ater). Instead, the young par- (1) Visitorsto the nest.Animals visiting the nest would asite’s advantage over nestmates is usually attributed to have an opportunity to damage eggs.Possible visitors in- its hatchingearlier than the hosts,to the preferential treat- cludeoredators and adult female cowbirds(Mavfield 1961). -
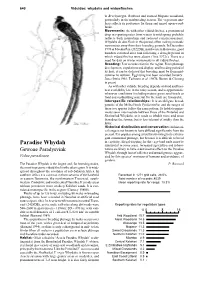
Paradise Whydah Substantial in Some Areas
640 Viduidae: whydahs and widowfinches in Brachystegia, Baikiaea and stunted Mopane woodland, particularly in the nonbreeding season. The vegetation ana- lysis reflects its preference for thorn and mixed open wood- lands. Movements: As with other viduid finches, a pronounced drop in reporting rates from winter to mid-spring probably reflects both nomadism and reduced conspicuousness. Whydahs do also flock in this period, often making nomadic movements away from their breeding grounds. In December 1970 at Mwaku Pan (2022DB), northwestern Botswana, good numbers returned after rain following a drought period in which viduid finches were absent (Tree 1972c). There is a need for data on winter movements in all viduid finches. Breeding: Few records exist for the region. From plumage development, copulations and display, and breeding period of its host, it can be deduced that breeding must be from mid- summer to autumn. Egglaying has been recorded January– June (Irwin 1981; Tarboton et al. 1987b; Brown & Clinning in press). As with other viduids, breeding depends on food and host- nest availability late in the rainy season, and is opportunistic whenever conditions (including mature green seed-heads as food and nestbuilding material for the host) are favourable. Interspecific relationships: It is an obligate brood- parasite of the Melba Finch Pytilia melba, and the ranges of these two species follow the same pattern. Its habitat require- ments seem intermediate between those of the Pintailed and Shafttailed Whydahs, as it tends to inhabit more arid areas than does the former, but is less tolerant of aridity than the latter. Historical distribution and conservation: Its histori- cal range is not known to have differed significantly from the present. -

Kenyan Birding & Animal Safari Organized by Detroit Audubon and Silent Fliers of Kenya July 8Th to July 23Rd, 2019
Kenyan Birding & Animal Safari Organized by Detroit Audubon and Silent Fliers of Kenya July 8th to July 23rd, 2019 Kenya is a global biodiversity “hotspot”; however, it is not only famous for extraordinary viewing of charismatic megafauna (like elephants, lions, rhinos, hippos, cheetahs, leopards, giraffes, etc.), but it is also world-renowned as a bird watcher’s paradise. Located in the Rift Valley of East Africa, Kenya hosts 1054 species of birds--60% of the entire African birdlife--which are distributed in the most varied of habitats, ranging from tropical savannah and dry volcanic- shaped valleys to freshwater and brackish lakes to montane and rain forests. When added to the amazing bird life, the beauty of the volcanic and lava- sculpted landscapes in combination with the incredible concentration of iconic megafauna, the experience is truly breathtaking--that the Africa of movies (“Out of Africa”), books (“Born Free”) and documentaries (“For the Love of Elephants”) is right here in East Africa’s Great Rift Valley with its unparalleled diversity of iconic wildlife and equatorially-located ecosystems. Kenya is truly the destination of choice for the birdwatcher and naturalist. Karibu (“Welcome to”) Kenya! 1 Itinerary: Day 1: Arrival in Nairobi. Our guide will meet you at the airport and transfer you to your hotel. Overnight stay in Nairobi. Day 2: After an early breakfast, we will embark on a full day exploration of Nairobi National Park--Kenya’s first National Park. This “urban park,” located adjacent to one of Africa’s most populous cities, allows for the possibility of seeing the following species of birds; Olivaceous and Willow Warbler, African Water Rail, Wood Sandpiper, Great Egret, Red-backed and Lesser Grey Shrike, Rosy-breasted and Pangani Longclaw, Yellow-crowned Bishop, Jackson’s Widowbird, Saddle-billed Stork, Cardinal Quelea, Black-crowned Night- heron, Martial Eagle and several species of Cisticolas, in addition to many other unique species. -

Species at Risk Act
Consultation on Amending the List of Species under the Species at Risk Act Terrestrial Species November 2011 Information contained in this publication or product may be reproduced, in part or in whole, and by any means, for personal or public non-commercial purposes, without charge or further permission, unless otherwise specified. You are asked to: Exercise due diligence in ensuring the accuracy of the materials reproduced; Indicate both the complete title of the materials reproduced, as well as the author organization; and Indicate that the reproduction is a copy of an official work that is published by the Government of Canada and that the reproduction has not been produced in affiliation with or with the endorsement of the Government of Canada. Commercial reproduction and distribution is prohibited except with written permission from the Government of Canada’s copyright administrator, Public Works and Government Services of Canada (PWGSC). For more information, please contact PWGSC at 613-996-6886 or at [email protected]. Cover photo credits: Olive Clubtail © Jim Johnson Peacock Vinyl Lichen © Timothy B. Wheeler Cerulean Warbler © Carl Savignac Title page photo credits: Background photo: Dune Tachinid Fly habitat © Sydney Cannings Foreground, large photo: Dwarf Lake Iris © Jessie M. Harris Small photos, left to right: Butler’s Gartersnake © Daniel W.A. Noble Hungerford’s Crawling Water Beetle © Steve Marshall Barn Swallow © Gordon Court Spring Salamander © David Green Available also on the Internet. ISSN: 1710-3029 Cat. no.: EN1-36/2011E-PDF © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2011 Consultation on Amending the List of Species under the Species at Risk Act Terrestrial Species November 2011 Please submit your comments by February 8, 2012, for terrestrial species undergoing normal consultations and by November 8, 2012, for terrestrial species undergoing extended consultations. -

Singapore Avifauna Volume 22 No 7 ______
SSIINNGGAAPPOORREE AAVVIIFFAAUUNNAA A monthly bulletin of the Nature Society (Singapore) Bird Group Volume 22 Published by Nature Society (Singapore) Bird Group, 510 Geylang Road, #02-05, The Sunflower, Singapore 389466. Number 7 Tel : 67412036, Fax : 67410871, Email : [email protected] , Website : http://www.nss.org.sg MICA(P) 239/11/2005 CONTENTS 1 Bird Report: July 2008 Compiled by Albert Low NSS Bird Group 9 Grey-headed Fish Eagle at “Little Guilin” by Ulf Remahl Chairman th Lim Kim Keang 11 Report on the 9 Mid-Year Bird Census by Lim Kim Seng ([email protected] ) 15 Notes on the Identification, Status and Distribution of Horsfield’s Vice-Chairman Bronze Cuckoo Chrysococcyx basalis in Singapore by Lim Kim Ho Hua Chew Seng ([email protected] ) Secretary SINAV Willie Foo ([email protected] ) Editorial Committee Lim Kim Chuah, Lim Kim Seng, Yong Ding Li, Andrew Chow, Albert Low Grey-headed Fish Eagle at ‘Little Guilin’ by Lau Weng Thor Nature Society (Singapore) is the national partner of Singapore Avifauna Volume 22 No 7 _____________________________________________________________________________ Bird Report July 2008 By Albert Low Highlights Singapore July is generally considered a quiet month in Singapore’s avian calendar, lodged in the birding no-man’s land between the end of the breeding season and the patient waiting on the part of birders and photographers for the year’s arrivals from the North to stream in. Thankfully, there was enough to keep both parties occupied this year. Of particular interest was the slew of Horsfield’s Bronze-cuckoo sightings early in the month. -

Short Communications
Short Communications The Wilson Journal of Ornithology 118(1):99±101, 2006 Provisioning of Fledgling Conspeci®cs by Males of the Brood-parasitic Cuckoos Chrysococcyx klaas and C. caprius Irby J. Lovette,1,4 Dustin R. Rubenstein,1,2,3 and Wilson Nderitu Watetu3 ABSTRACT.ÐAlthough post-¯edging care by adult Over the past century, there have been nu- males seems unlikely in bird species that are obligate, merous observations of male Chrysococcyx interspeci®c brood parasites, there have been numer- cuckoos feeding conspeci®cs that were ous reports of adult male Chrysococcyx cuckoos ap- parently feeding conspeci®c young. Most researchers thought to be ¯edglings (Moreau 1944, Fried- currently view these observations with skepticism, in mann 1968, Iversen and Hill 1983, Rowan large part because Chrysococcyx and other cuckoo spe- 1983). In a literature review of provisioning cies engage in courtship feeding, and it is possible that behavior in brood parasites, Lorenzana and ®eld observers could mistake adult females receiving Sealy (1998) found 5 records of nestling or food from courting males for ¯edglings, especially giv- en the similar appearances of females and juveniles. ¯edgling provisioning by Klaas's Cuckoo Here, we report an observation of an extended provi- males and 11 such records for Diederik Cuck- sioning bout by an adult male Klaas's Cuckoo (C. oo males; Friedmann (1968) discusses 12 and klaas) feeding a conspeci®c individual with juvenile 15 such records, respectively, including some plumage and behavior, and we summarize our obser- anecdotal reports. There is apparently only vations of similar occurrences in the Diederik Cuckoo one equivalent report of a female Chrysococ- (C. -

Chrysococcyx Lucidus) in New Zealand
249 Notornis, 2013, Vol. 60: 249-251 0029-4470 © The Ornithological Society of New Zealand, Inc. SHORT NOTE Observation of food presentation behaviour between individual shining cuckoos (Chrysococcyx lucidus) in New Zealand M.N.H. SEABROOK-DAVISON* M.G. ANDERSON Ecology, Behaviour and Conservation Group, Institute of Natural and Mathematical Sciences, Massey University, Private Bag 102904, Auckland 0745, New Zealand Little is known about courtship behaviour in the ensure a rapid onset of breeding condition in female shining cuckoo (Chrysococcyx lucidus) (also known cuckoos. as Chalcites lucidus) and whether males feed females The shining cuckoo is an obligate specialist brood as occurs in a number of other species. Although parasite that only uses the grey warbler (Gerygone there have been a number of observations of male igata) as a host species on the main islands of New Chrysococcyx cuckoos feeding suspected conspecific Zealand (Heather & Robertson 1996; Gill 1983b) and fledglings (Moreau 1944; Friedmann 1968; Iversen the Chatham Island warbler (Gerygone albofrontata) & Hill 1983; Lovette et al. 2006), more recently it on the Chatham Is (Dennison et al. 1984). Little has been suggested that these were misdirected has been recorded of the courtship behaviour of courtship feeding or the observers misidentified the shining cuckoo. Seabrook-Davison et al. (2008) adult female cuckoos as fledglings (Lorenzana described pre-copulatory behaviour and copulation & Sealy 1998; Davies 2000). If this is the case, in a pair of shining cuckoos at Coatesville, 28 km these observations may be indicative of courtship north of Auckland, New Zealand. This behaviour behaviours and that courtship feeding occurs more was observed only once during 5 minute bird-count frequently than previous appreciated.