Nest Predation by Brown-Headed Cowbirds (Molothrus Ater)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lista Roja De Las Aves Del Uruguay 1
Lista Roja de las Aves del Uruguay 1 Lista Roja de las Aves del Uruguay Una evaluación del estado de conservación de la avifauna nacional con base en los criterios de la Unión Internacional para la Conservación de la Naturaleza. Adrián B. Azpiroz, Laboratorio de Genética de la Conservación, Instituto de Investigaciones Biológicas Clemente Estable, Av. Italia 3318 (CP 11600), Montevideo ([email protected]). Matilde Alfaro, Asociación Averaves & Facultad de Ciencias, Universidad de la República, Iguá 4225 (CP 11400), Montevideo ([email protected]). Sebastián Jiménez, Proyecto Albatros y Petreles-Uruguay, Centro de Investigación y Conservación Marina (CICMAR), Avenida Giannattasio Km 30.5. (CP 15008) Canelones, Uruguay; Laboratorio de Recursos Pelágicos, Dirección Nacional de Recursos Acuáticos, Constituyente 1497 (CP 11200), Montevideo ([email protected]). Cita sugerida: Azpiroz, A.B., M. Alfaro y S. Jiménez. 2012. Lista Roja de las Aves del Uruguay. Una evaluación del estado de conservación de la avifauna nacional con base en los criterios de la Unión Internacional para la Conservación de la Naturaleza. Dirección Nacional de Medio Ambiente, Montevideo. Descargo de responsabilidad El contenido de esta publicación es responsabilidad de los autores y no refleja necesariamente las opiniones o políticas de la DINAMA ni de las organizaciones auspiciantes y no comprometen a estas instituciones. Las denominaciones empleadas y la forma en que aparecen los datos no implica de parte de DINAMA, ni de las organizaciones auspiciantes o de los autores, juicio alguno sobre la condición jurídica de países, territorios, ciudades, personas, organizaciones, zonas o de sus autoridades, ni sobre la delimitación de sus fronteras o límites. -

Nest Building and Nesting Behavior of the Brown Cacholote
Wilson Bull., 106(l), 1994, pp. 106-120 NEST BUILDING AND NESTING BEHAVIOR OF THE BROWN CACHOLOTE ANA I. NOBES AND MANUEL NORES ’ ABsTnAcr.-we studied nesting behavior ofthe Brown Cacholote (Pseudoseisuralophotes) in Cordoba, Argentina from April 1989 to March 1993. Brown Cacholotes build many elaborate stick nests throughout the year and use each of them during the breeding period or for a short time in the non-breeding period. Nest building requires 15 to 37 days. Nests were usually built with thorny twigs, but the nature of the materials depends on availability. Usually each pair had several nests or part of them in their territory (range = l-10). Nest building requires much of the birds ’ time and energy, but Brown Cacholotes generally use the material of old nests to minimize energy expenditure in nest construction. Both sexes shared all nesting activities. The birds copulate inside the nest, which is apparently unknown among birds. Egg laying occurred from last September to late February. Mean clutch size was 2.6 eggs (range = 2-4). The incubation period lasted 18-20 days and the nestling period 18-23 days. Nesting success was 59.3%, and an average of 1.5 nestlings were reared per clutch. Parental breeding experience, rather than age, would be more important influence on clutch-size and nesting success. Juveniles remained in the parental territories for 4-l 3 months. They contributed to nest building and defense of their territory, but their help was minimal. Received7 Dec. 1992, acceptedI Sept. 1993. Although nest has been defined as a structure that aids the development of the eggs and the survival of young (Collias 1964) some birds build similar or different structures for use as dormitories throughout the year and have a close relation with them (Skutch 196 1, Collias 1964, Welty 1979). -

Coversheet for Thesis in Sussex Research Online
A University of Sussex DPhil thesis Available online via Sussex Research Online: http://sro.sussex.ac.uk/ This thesis is protected by copyright which belongs to the author. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the Author The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the Author When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given Please visit Sussex Research Online for more information and further details Information gathering and conflict resolution in Polistes wasps Jonathan Philip Green Submitted for the degree of Doctor of Philosophy University of Sussex September 2011 ii Declaration The design and data collection for the study presented in Chapter 4 were undertaken in collaboration with Dr. Elli Leadbeater at the Institute of Zoology and Professor Jeremy Field at the University of Sussex. However, the particular analyses undertaken in that chapter, as well as the interpretations drawn from the data, are my own. I certify that, with the above qualification, the work carried out in this thesis is entirely my own, and that any help provided by other individuals with data collection and analysis is fully acknowledged. In addition, I certify that this thesis has not been, and will not be, submitted in whole or in part to another university for the award of any other degree. Signature: Jonathan Philip Green iii UNIVERISTY OF SUSSEX JONATHAN PHILIP GREEN, DOCTOR OF PHILOSOPHY INFORMATION GATHERING AND CONFLICT RESOLUTION IN POLISTES WASPS SUMMARY Signals are used to communicate resource-holding potential (RHP) to rivals during contests across a wide range of taxa. -

A New Species of the Paper Wasp Genus Polistes (Hymenoptera, Vespidae, Polistinae) in Europe Revealed by Morphometrics and Molecular Analyses
A peer-reviewed open-access journal ZooKeys 400:A 67–118new species (2014) of the paper wasp genus Polistes (Hymenoptera, Vespidae, Polistinae)... 67 doi: 10.3897/zookeys.400.6611 RESEARCH ARTICLE www.zookeys.org Launched to accelerate biodiversity research A new species of the paper wasp genus Polistes (Hymenoptera, Vespidae, Polistinae) in Europe revealed by morphometrics and molecular analyses Rainer Neumeyer1,†, Hannes Baur2,‡, Gaston-Denis Guex3,§, Christophe Praz4,| 1 Probsteistrasse 89, CH-8051 Zürich, Switzerland 2 Abteilung Wirbellose Tiere, Naturhistorisches Museum der Burgergemeinde Bern, Bernastrasse 15, CH-3005 Bern, Switzerland 3 Institute of Evolutionary Biology and Environmental Studies, Field Station Dätwil, University of Zurich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland 4 Evolutionary Entomology, Institute of Biology, University of Neuchatel, Emile-Argand 11, CH-2000 Neuchâtel, Switzerland † http://zoobank.org/B0BFC898-8FDF-4E01-8657-CBADE08292D3 ‡ http://zoobank.org/76BB8FCD-3EC9-4774-8FF3-194D57A8A905 § http://zoobank.org/D038CAA8-4662-4BD9-931A-C0FD5FC623D8 | http://zoobank.org/0718546C-5D1A-44D4-921C-C7EA0C11BE0A Corresponding author: Rainer Neumeyer ([email protected]) Academic editor: M. Buffington | Received 13 November 2013 | Accepted 18 March 2014 | Published 11 April 2014 http://zoobank.org/91DC4784-F49A-4353-B4C8-DC0F67B1EF92 Citation: Neumeyer R, Baur H, Guex G-D, Praz C (2014) A new species of the paper wasp genus Polistes (Hymenoptera, Vespidae, Polistinae) in Europe revealed by morphometrics and molecular analyses. ZooKeys 400: 67–118. doi: 10.3897/ zookeys.400.6611 Abstract We combine multivariate ratio analysis (MRA) of body measurements and analyses of mitochondrial and nuclear data to examine the status of several species of European paper wasps (Polistes Latreille, 1802) closely related to P. -

Prairie Ridge Species Checklist 2018
Prairie Ridge Species Checklist Genus species Common Name Snails Philomycus carolinianus Carolina Mantleslug Gastrocopta contracta Bottleneck Snaggletooth Glyphalinia wheatleyi Bright Glyph Triodopsis hopetonensis Magnolia Threetooth Triodopsis juxtidens Atlantic Threetooth Triodopsis fallax Mimic Threetooth Ventridens cerinoideus Wax Dome Ventridens gularis Throaty Dome Anguispira fergusoni Tiger Snail Zonitoides arboreus Quick Gloss Deroceras reticulatum Gray Garden Slug Mesodon thyroidus White-lip Globe Slug Stenotrema stenotrema Inland Stiltmouth Melanoides tuberculatus Red-rim Melania Spiders Argiope aurantia Garden Spider Peucetia viridans Green Lynx Spider Phidippus putnami Jumping Spider Phidippus audax Jumping Spider Phidippus otiosus Jumping Spider Centipedes Hemiscolopendra marginata Scolopocryptops sexspinosus Scutigera coleoptrata Geophilomorpha Millipedes Pseudopolydesmus serratus Narceus americanus Oxidus gracilis Greenhouse Millipede Polydesmidae Crayfishes Cambarus “acuminatus complex” (= “species C”) Cambarus (Depressicambarus) latimanus Cambarus (Puncticambarus) (="species C) Damselflies Calopteryx maculata Ebony Jewelwing Lestes australis Southern Spreadwing Lestes rectangularis Slender Spreadwing Lestes vigilax Swamp Spreadwing Lestes inaequalis Elegant Spreadwing Enallagma doubledayi Atlantic Bluet Enallagma civile Familiar Bluet Enallagma aspersum Azure Bluet Enallagma exsulans Stream Bluet Enallegma signatum Orange Bluet Ischnura verticalis Eastern Forktail Ischnura posita Fragile Forktail Ischnura hastata Citrine -

N a T U R E P R O G R a M S F I E L D T R I
EASTERN LONG ISLAND AUDUBON SOCIETY – From the Barrens to the Bays Formerly Moriches Bay Audubon, established 1967 November/December 2008 —Vol. XXXVIII No. 6 NATURE PROGRAMS J a Monday, November 3rd m e s NY'S SECOND BREEDING BIRD ATLAS G a l l BY KIMBERLEY CORWIN e t t o NewYork’s second Breeding Bird Atlas is imminent. The book documents changes in our bird distributions over the past 20 years. Some species have increased greatly while others have declined alarmingly. A few species are new breeders in the state while at least one has all but disappeared. Kimberley Corwin, who coordinated the Atlas project and co-edited the Atlas pub - lication, will show distribution maps and share some of the stories with us. Get a sneak peak into the book that NewYork's birders have been waiting years to see! The first feeding from A RedTale Kim is the Co-Editor of the Breeding Bird Atlas publication. She served as the Monday, December 1st James Galletto has been photographing Project Coordinator of the project from JAMES GALLETTO - A RED TALE nature for ten years and is known world its start in 1999. Kim holds a Master's de - wide for his action and behavior photog - James Galletto will give a program called gree in Ecology and Evolutionary Biology raphy. His images have graced the cover of A RedTale an intimate view inside the from the University at Albany. In her spare Natures Best Magazine and have hung on life of a Red-tail Hawk Family. We will fol - time, Kim enjoys hiking and birding. -

Brown-Headed Cowbird (Molothrus Ater) Doug Powless
Brown-headed Cowbird (Molothrus ater) Doug Powless Grandville, Kent Co., MI. 5/4/2008 © John Van Orman (Click to view a comparison of Atlas I to II) Brown-headed Cowbirds likely flourished Distribution Brown-headed Cowbirds breed in grassland, alongside the Pleistocene megafauna that once prairie, and agricultural habitats across southern roamed North America (Rothstein and Peer Canada to Florida, the Gulf of Mexico, and 2005). In modern times, flocks of cowbirds south into central Mexico (Lowther 1993). The followed the great herds of bison across the center of concentration and highest abundance grasslands of the continent, feeding on insects of the cowbird during summer occurs in the kicked up, and depositing their eggs in other Great Plains and Midwestern prairie states birds’ nests along the way. An obligate brood where herds of wild bison and other ungulates parasite, the Brown-headed Cowbird is once roamed (Lowther 1993, Chace et al. 2005). documented leaving eggs in the nests of hundreds of species (Friedmann and Kiff 1985, Wild bison occurred in southern Michigan and Lowther 1993). The evolution of this breeding across forest openings in the East until about strategy is one of the most fascinating aspects of 1800 before being hunted to near-extinction North American ornithology (Lanyon 1992, across the continent (Baker 1983, Kurta 1995). Winfree 1999, Rothstein et al. 2002), but the Flocks of cowbirds likely also inhabited the cowbird has long drawn disdain. Chapman prairies and woodland openings of southern (1927) called it “. a thoroughly contemptible Michigan prior to the 1800s (Walkinshaw creature, lacking in every moral and maternal 1991). -

Rejection Behavior by Common Cuckoo Hosts Towards Artificial Brood Parasite Eggs
REJECTION BEHAVIOR BY COMMON CUCKOO HOSTS TOWARDS ARTIFICIAL BROOD PARASITE EGGS ARNE MOKSNES, EIVIN ROSKAFT, AND ANDERS T. BRAA Departmentof Zoology,University of Trondheim,N-7055 Dragvoll,Norway ABSTRACT.--Westudied the rejectionbehavior shown by differentNorwegian cuckoo hosts towardsartificial CommonCuckoo (Cuculus canorus) eggs. The hostswith the largestbills were graspejectors, those with medium-sizedbills were mostlypuncture ejectors, while those with the smallestbills generally desertedtheir nestswhen parasitizedexperimentally with an artificial egg. There were a few exceptionsto this general rule. Becausethe Common Cuckooand Brown-headedCowbird (Molothrus ater) lay eggsthat aresimilar in shape,volume, and eggshellthickness, and they parasitizenests of similarly sizedhost species,we support the punctureresistance hypothesis proposed to explain the adaptivevalue (or evolution)of strengthin cowbirdeggs. The primary assumptionand predictionof this hypothesisare that somehosts have bills too small to graspparasitic eggs and thereforemust puncture-eject them,and that smallerhosts do notadopt ejection behavior because of the heavycost involved in puncture-ejectingthe thick-shelledparasitic egg. We comparedour resultswith thosefor North AmericanBrown-headed Cowbird hosts and we found a significantlyhigher propor- tion of rejectersamong CommonCuckoo hosts with graspindices (i.e. bill length x bill breadth)of <200 mm2. Cuckoo hosts ejected parasitic eggs rather than acceptthem as cowbird hostsdid. Amongthe CommonCuckoo hosts, the costof acceptinga parasiticegg probably alwaysexceeds that of rejectionbecause cuckoo nestlings typically eject all hosteggs or nestlingsshortly after they hatch.Received 25 February1990, accepted 23 October1990. THEEGGS of many brood parasiteshave thick- nestseither by grasping the eggs or by punc- er shells than the eggs of other bird speciesof turing the eggs before removal. Rohwer and similar size (Lack 1968,Spaw and Rohwer 1987). -
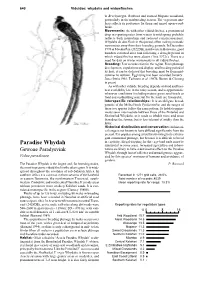
Paradise Whydah Substantial in Some Areas
640 Viduidae: whydahs and widowfinches in Brachystegia, Baikiaea and stunted Mopane woodland, particularly in the nonbreeding season. The vegetation ana- lysis reflects its preference for thorn and mixed open wood- lands. Movements: As with other viduid finches, a pronounced drop in reporting rates from winter to mid-spring probably reflects both nomadism and reduced conspicuousness. Whydahs do also flock in this period, often making nomadic movements away from their breeding grounds. In December 1970 at Mwaku Pan (2022DB), northwestern Botswana, good numbers returned after rain following a drought period in which viduid finches were absent (Tree 1972c). There is a need for data on winter movements in all viduid finches. Breeding: Few records exist for the region. From plumage development, copulations and display, and breeding period of its host, it can be deduced that breeding must be from mid- summer to autumn. Egglaying has been recorded January– June (Irwin 1981; Tarboton et al. 1987b; Brown & Clinning in press). As with other viduids, breeding depends on food and host- nest availability late in the rainy season, and is opportunistic whenever conditions (including mature green seed-heads as food and nestbuilding material for the host) are favourable. Interspecific relationships: It is an obligate brood- parasite of the Melba Finch Pytilia melba, and the ranges of these two species follow the same pattern. Its habitat require- ments seem intermediate between those of the Pintailed and Shafttailed Whydahs, as it tends to inhabit more arid areas than does the former, but is less tolerant of aridity than the latter. Historical distribution and conservation: Its histori- cal range is not known to have differed significantly from the present. -

Changes in the Insect Fauna of a Deteriorating Riverine Sand Dune
., CHANGES IN THE INSECT FAUNA OF A DETERIORATING RIVERINE SAND DUNE COMMUNITY DURING 50 YEARS OF HUMAN EXPLOITATION J. A. Powell Department of Entomological Sciences University of California, Berkeley May , 1983 TABLE OF CONTENTS INTRODUCTION 1 HISTORY OF EXPLOITATION 4 HISTORY OF ENTOMOLOGICAL INVESTIGATIONS 7 INSECT FAUNA 10 Methods 10 ErRs s~lected for compar"ltive "lnBlysis 13 Bio1o~ica1 isl!lnd si~e 14 Inventory of sp~cies 14 Endemism 18 Extinctions 19 Species restricted to one of the two refu~e parcels 25 Possible recently colonized species 27 INSECT ASSOCIATES OF ERYSIMUM AND OENOTHERA 29 Poll i n!ltor<'l 29 Predqt,.n·s 32 SUMMARY 35 RECOm1ENDATIONS FOR RECOVERY ~4NAGEMENT 37 ACKNOWT.. EDGMENTS 42 LITERATURE CITED 44 APPENDICES 1. T'lbles 1-8 49 2. St::ttns of 15 Antioch Insects Listed in Notice of 75 Review by the U.S. Fish "l.nd Wildlife Service INTRODUCTION The sand dune formation east of Antioch, Contra Costa County, California, comprised the largest riverine dune system in California. Biogeographically, this formation was unique because it supported a northern extension of plants and animals of desert, rather than coastal, affinities. Geologists believe that the dunes were relicts of the most recent glaciation of the Sierra Nevada, probably originating 10,000 to 25,000 years ago, with the sand derived from the supratidal floodplain of the combined Sacramento and San Joaquin Rivers. The ice age climate in the area is thought to have been cold but arid. Presumably summertime winds sweeping through the Carquinez Strait across the glacial-age floodplains would have picked up the fine-grained sand and redeposited it to the east and southeast, thus creating the dune fields of eastern Contra Costa County. -

Rote Liste Der Faltenwespen / 2013 / 1. Fassung
Hessisches Ministerium für Umwelt, Energie, Landwirtschaft und Verbraucherschutz Rote Liste der Faltenwespen Hessens (Hymenoptera Vespidae: Eumeninae, Polistinae, Vespinae) Rote Liste der Faltenwespen Hessens (Hymenoptera Vespidae: Eumeninae, Polistinae, Vespinae) 1. Fassung (Stand 6. 6. 2013) Stefan Tischendorf, Karl-Heinz Schmalz, Hans-Joachim Flügel, Ulrich Frommer, Wolfgang H. O. Dorow, Franz Malec im Auftrag des Hessischen Ministeriums für Umwelt, Energie, Landwirtschaft und Verbraucherschutz (HMUELV). Inhaltsübersicht Zusammenfassung ................................................................................................. 3 Einleitung ................................................................................................................. 4 Faltenwespen .......................................................................................................... 5 Systematik ............................................................................................................ 5 Artenzahlen und Gefährdung in Deutschland ...................................................... 5 Lebensraum, Nistweise und Beutetiere ................................................................ 6 Stand der Erforschung der Faltenwespenfauna in Hessen .................................. 7 Material und Methoden ........................................................................................... 9 Grundlagen zur Einstufung nach BfN-Kriterien .................................................... 9 Kategorien und Eicharten .................................................................................... -

The Shiny Cowbird Reaches the United States
BREEDING RECORD The ShinyCowbird reaches the UnitedStates Will the scourge of the Caribbean impact Florida's avifauna too? P. William Smith and Alexander Sprunt IV ductinga BreedingBird Surveyon NLowerJUNE Matecumbe14,1985, Key,WHILE MonroeCON- County, Florida, Sprunt and Karen Sunderland discoveredthe first Shiny Cowbird (Molothrus bonariensis) re- ported in the United States,a male, with Red-winged Blackbirds (Agelaius phoeniceus) in mangroves near the shoulderof the OverseasHighway (U.S. 1). Its appearancein Florida was hardly unexpected. From its South American base, the Shiny Cowbird has spread northward, mostly during this century, through the Antilles; its expansionhas been particularly strongover the last 30 years(Post and Wiley 1977; Arendt and Figure 1. Three male Shiny Cowbirdsjoin Mourning Dovesand Eura•ian Collared-Dovesat Vargas 1984). By 1982, the specieshad Islamorada.Florida. July 5, 1986. Photo/P William Smith (VIREO SI4/2/003). reachedthe north coastof Cuba (Bond in the United States. This constituted On the other hand, female and ju- 1984;Cruz el al. 1985), only about 100 the first recordfor the Florida peninsula venile Shiny Cowbirds representa def- miles south of its point of discoveryin and the first definite report of a female inite identification challenge (Figs. 5- the United States. After it was relocated in the United States. Up to five male 6). An examination of specimensat the at a nearby feeder, the Lower Matec- and two female Shinies were seen to- Academy of Natural Sciences,in Phil- umbe Shiny Cowbird was observedby getherwith the Brown-headedCowbirds adelphia, reveals that except for head birders for several weeks. at Flamingo until at least late June and bill shape,there is almost complete In 1986, Smith and his wife were 1987.A juvenileShiny was present from overlap in charactersbetween female trying to capture Eurasian Collared- June 27 until late July, suggestingthe Shinies of the race minimus.