Investigations of Evolutionary Arms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lista Roja De Las Aves Del Uruguay 1
Lista Roja de las Aves del Uruguay 1 Lista Roja de las Aves del Uruguay Una evaluación del estado de conservación de la avifauna nacional con base en los criterios de la Unión Internacional para la Conservación de la Naturaleza. Adrián B. Azpiroz, Laboratorio de Genética de la Conservación, Instituto de Investigaciones Biológicas Clemente Estable, Av. Italia 3318 (CP 11600), Montevideo ([email protected]). Matilde Alfaro, Asociación Averaves & Facultad de Ciencias, Universidad de la República, Iguá 4225 (CP 11400), Montevideo ([email protected]). Sebastián Jiménez, Proyecto Albatros y Petreles-Uruguay, Centro de Investigación y Conservación Marina (CICMAR), Avenida Giannattasio Km 30.5. (CP 15008) Canelones, Uruguay; Laboratorio de Recursos Pelágicos, Dirección Nacional de Recursos Acuáticos, Constituyente 1497 (CP 11200), Montevideo ([email protected]). Cita sugerida: Azpiroz, A.B., M. Alfaro y S. Jiménez. 2012. Lista Roja de las Aves del Uruguay. Una evaluación del estado de conservación de la avifauna nacional con base en los criterios de la Unión Internacional para la Conservación de la Naturaleza. Dirección Nacional de Medio Ambiente, Montevideo. Descargo de responsabilidad El contenido de esta publicación es responsabilidad de los autores y no refleja necesariamente las opiniones o políticas de la DINAMA ni de las organizaciones auspiciantes y no comprometen a estas instituciones. Las denominaciones empleadas y la forma en que aparecen los datos no implica de parte de DINAMA, ni de las organizaciones auspiciantes o de los autores, juicio alguno sobre la condición jurídica de países, territorios, ciudades, personas, organizaciones, zonas o de sus autoridades, ni sobre la delimitación de sus fronteras o límites. -
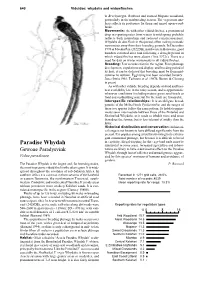
Paradise Whydah Substantial in Some Areas
640 Viduidae: whydahs and widowfinches in Brachystegia, Baikiaea and stunted Mopane woodland, particularly in the nonbreeding season. The vegetation ana- lysis reflects its preference for thorn and mixed open wood- lands. Movements: As with other viduid finches, a pronounced drop in reporting rates from winter to mid-spring probably reflects both nomadism and reduced conspicuousness. Whydahs do also flock in this period, often making nomadic movements away from their breeding grounds. In December 1970 at Mwaku Pan (2022DB), northwestern Botswana, good numbers returned after rain following a drought period in which viduid finches were absent (Tree 1972c). There is a need for data on winter movements in all viduid finches. Breeding: Few records exist for the region. From plumage development, copulations and display, and breeding period of its host, it can be deduced that breeding must be from mid- summer to autumn. Egglaying has been recorded January– June (Irwin 1981; Tarboton et al. 1987b; Brown & Clinning in press). As with other viduids, breeding depends on food and host- nest availability late in the rainy season, and is opportunistic whenever conditions (including mature green seed-heads as food and nestbuilding material for the host) are favourable. Interspecific relationships: It is an obligate brood- parasite of the Melba Finch Pytilia melba, and the ranges of these two species follow the same pattern. Its habitat require- ments seem intermediate between those of the Pintailed and Shafttailed Whydahs, as it tends to inhabit more arid areas than does the former, but is less tolerant of aridity than the latter. Historical distribution and conservation: Its histori- cal range is not known to have differed significantly from the present. -

Manyoni Private Game Reserve (Previously Zululand Rhino Reserve)
Manyoni Private Game Reserve (Previously Zululand Rhino Reserve) Gorgeous Bushshrike by Adam Riley BIRD LIST Prepared by Adam Riley [email protected] • www.rockjumperbirding. -

Adult Brood Parasites Feeding Nestlings and Fledglings of Their Own Species: a Review
J. Field Ornithol., 69(3):364-375 ADULT BROOD PARASITES FEEDING NESTLINGS AND FLEDGLINGS OF THEIR OWN SPECIES: A REVIEW JANICEC. LORENZANAAND SPENCER G. SEALY Departmentof Zoology Universityof Manitoba Winnipeg,Manitoba R3T 2N2 Canada Abstract.--We summarized 40 reports of nine speciesof brood parasitesfeeding young of their own species.These observationssuggest that the propensityto provisionyoung hasnot been lost entirely in brood parasitesdespite the belief that brood parasiticadults abandon their offspringat the time of laying.The hypothesisthat speciesthat participatein courtship feeding are more likely to provisionyoung was not supported:provisioning of young has been observedin two speciesof brood parasitesthat do not courtshipfeed. The function of this provisioningis unknown, but we suggestit may be: (1) a non-adaptivevestigial behavior or (2) an adaptation to ensure adequatecare of parasiticyoung. The former is more likely the case.Further studiesare required to determinewhether parasiticadults commonly feed their genetic offspring. ADULTOS DE AVES PARAS•TICASALIMENTANDO PICHONES Y VOLANTONES DE SU PROPIA ESPECIE: UNA REVISION Sinopsis.--Resumimos40 informes de nueve especiesde avesparasiticas que alimenaron a pichonesde su propia especie.Las observacionessugieren que la propensividadde alimentar a los pichonesno ha sido totalmente perdida en las avesparasiticas, no empecea la creencia de que los parasiticosabandonan su progenie al momento de poner los huevos.La hipttesis de que las especiesque participan en cortejo de alimentacitn, son milspropensas a alimentar los pichonesno tuvo apoyo.Las observacionesde alimentacitn a pichonesse han hecho en dos especiesparasiticas cuyo cortejo no incluye la alimentacitn de la pareja. La funcitn de proveer alimento se desconoce.No obstante,sugerimos que pueda ser: 1) una conducta vestigialno adaptativa,o 2) una adaptacitn parc asegurarel cuidado adecuadode los pi- chonesparasiticos. -

Screaming Cowbird Parasitism of Nests of Solitary Caciques and Cattle Tyrants
SHORT COMMUNICATIONS 795 The Wilson Journal of Ornithology 122(4):795–799, 2010 Screaming Cowbird Parasitism of Nests of Solitary Caciques and Cattle Tyrants Alejandro G. Di Giacomo,1 Bettina Mahler,2 and Juan C. Reboreda2,3 ABSTRACT.—The Screaming Cowbird (Molothrus these events of parasitism resulted from recognition rufoaxillaris) is one of the most specialized brood errors by Screaming Cowbird females that regularly parasites with only three known hosts: Baywing parasitize Baywings and Chopi Blackbirds. The nest of (Agelaioides badius), the main host throughout most Solitary Caciques had been frequently visited by a pair of its range, and two alternative hosts in some areas of of Baywings before Screaming Cowbird parasitism its distribution, Chopi Blackbird (Gnorimopsar chopi) occurred, and the nest of Cattle Tyrants was near an and Brown-and-yellow Marshbird (Pseudoleistes vir- active Chopi Blackbird nest that had been previously escens). We studied Screaming Cowbird parasitism in parasitized by Screaming Cowbirds. Received 5 Janu- northeast Argentina where this parasite uses Baywings ary 2010. Accepted 7 April 2010. and Chopi Blackbirds as hosts. We monitored 69 nests of Baywings, 251 of Chopi Blackbirds, 31 of Solitary Caciques (Cacicus solitarius), and 30 of Cattle Tyrants (Machetornis rixosa). The frequency of Screaming The Screaming Cowbird (Molothrus rufoaxil- Cowbird parasitism on Baywing nests was 80% and laris) is a specialized brood parasite, which was 46% for Chopi Blackbirds. We recorded one event exclusively uses the Baywing (Agelaioides ba- of Screaming Cowbird parasitism on one nest of dius) as a host throughout most of its range Solitary Caciques and three events of Screaming (Mason 1980, Fraga 1998). -

Egg Laying Behavior of Common Cuckoos (Cuculus Canorus): Data Based on Field Video-Recordings
ZOOLOGICAL RESEARCH Egg laying behavior of common cuckoos (Cuculus canorus): Data based on field video-recordings DEAR EDITOR, terms of time, some parasites, such as common cuckoos, are The egg laying behavior of brood parasites is at the heart of known to lay their eggs in the afternoon or evening (Nakamura studies on host co-evolution. Therefore, research on egg et al., 2005; Wyllie, 1981). As hosts lay eggs and stay close to laying behavior can improve our understanding of brood the nest in the morning, but rarely in the afternoon, afternoon parasitism and associated processes. Over a seven year parasitism reduces the risk of detection by the host and the study period, we monitored 455 oriental reed warbler possibility of parasitic egg rejection (Davies & de L. Brooke, (Acrocephalus orientalis) nests during the egg laying period, 1988). However, some parasites such as cowbirds (Molothrus 250 of which were parasitized by common cuckoos (Cuculus spp.) complete egg laying before sunrise (Gloag et al., 2013; canorus). We collected 53 clear videos of common cuckoo Sealy, 1992). The entire process of egg laying by a brood parasitism, analyzed all recorded parasitic behavior in detail, parasite is usually short (less than 10 s) (Davies & de L. and summarized the process of brood parasitism. Brooke, 1988; Ellisson et al., 2020; Moksnes et al., 2000; Furthermore, based on analyses of the field video recordings, Scardamaglia et al., 2017; Soler & Soler, 2000); despite this, we propose a new explanation for egg removal behavior, in addition to egg laying, brood parasites also display other namely the delivery hypothesis, i.e., egg pecking and biting by complex behaviors. -

1 Convergent Evolution of Reduced Eggshell Conductance in Avian
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Royal Holloway - Pure Convergent evolution of reduced eggshell conductance in avian brood parasites Stephanie C. McClelland1*, Gabriel A. Jamie2,3, Katy S. Waters1, Lara Caldas1, Claire N. Spottiswoode2,3 and Steven J. Portugal1 1School of Biological Sciences, Royal Holloway University of London, Egham, Surrey, TW20 0EX, UK. 2Department of Zoology, University of Cambridge, Downing Street, University of 10 Cambridge, Cambridge CB2 3EJ, UK. 3FitzPatrick Institute of African Ornithology, DST-NRF Centre of Excellence, University of Cape Town, Rondebosch 7701, Cape Town, South Africa. Subject Areas: evolution, parasitism, physiology Keywords: Cuculidae, eggs, gas exchange, Indicatoridae, Viduidae Author for Correspondence: Stephanie C. McClelland, email: [email protected] 20 1 Abstract Brood parasitism has evolved independently in several bird lineages, yet these species 30 share many similar physiological traits that optimise this breeding strategy, such as shorter incubation periods and thicker eggshells. Eggshell structure is important for embryonic development as it controls the flux of metabolic gases, such as O2, CO2 and H2O, into and out of the egg; in particular, water vapour conductance (GH2O) is an essential process for optimal development of the embryo. Previous work has shown that common cuckoos (Cuculus canorus) have a lower than expected eggshell GH2O compared to their hosts. Here we sought to test whether this is a trait found in other independently-evolved avian brood parasites, and therefore reflects a general adaptation to a parasitic lifestyle. We analysed GH2O for seven species of brood parasites from four unique lineages as well as for their hosts, and combined this with species 40 from the literature. -

Evoluation of Brood Parasitism in Altricial Birds
THE CONDOR VOLUME 67 SEPTEMBER-OCTOBER NUMBER 4 EVOLUTION OF BROOD PARASITISM IN ALTRICIAL BIRDS BY WILLIAM J. HAMILTON, III, and GORDON H. ORIANS Among the 8600 living species of birds there are about 75 brood parasites which make no nest, laying their eggs in the nests of other birds. Here we wish to examine the behavior patterns and environmental circumstances which may have led to the development of this form of parasitism. This will involve comparisons with the adap- tations of nonparasitic relatives of modern brood parasites. Thus, we are attempting to evaluate not only why modern brood parasites may have developed their unusual habits but also why certain close relatives have not. Brood parasitism has evolved independently a number of times in birds. Best known are the cuckoos, with a complex of Old World species (and three less special- ized New World species) highly specialized in the parasitic habit (Friedmann, 1933, 1948). Also, all the African honey guides (Indicatoridae) whose breeding biology is known are brood parasites (Friedmann, 1955). Among passerines brood parasitism has evolved independently in African weaver birds (Ploceidae) and in several species of blackbirds (Icteridae) . The only obligate parasite in precocial species is the South American Black-headed Duck (Hetermetta atricupilZu), but the North American Redhead (Aythyu americana) has populations at various stages of parasitism, from completely independent to complete brood parasitism (Weller, 1959). The inde- pendent evolution of brood parasitism in these diverse phyletic lines is clear. But brood parasitism has not necessarily evolved only once in each group. The poly- phyletic origin of such a specialized habit within a group might seem quite improb- able, but the conditions which might lead to the development of such a habit sug- gest that this is a possibility. -

Southern Tanzania: Endemic Birds & Spectacular Mammals
SOUTHERN TANZANIA: ENDEMIC BIRDS & SPECTACULAR MAMMALS SEPTEMBER 18–OCTOBER 6, 2018 Tanzanian Red-billed Hornbill © Kevin J. Zimmer LEADERS: KEVIN ZIMMER & ANTHONY RAFAEL LIST COMPILED BY: KEVIN ZIMMER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM SOUTHERN TANZANIA: ENDEMIC BIRDS & SPECTACULAR MAMMALS September 18–October 6, 2018 By Kevin Zimmer After meeting in Dar es Salaam, we kicked off our inaugural Southern Tanzania tour by taking a small charter flight to Ruaha National Park, at 7,800 square miles, the largest national park in all of east Africa. The scenery from the air was spectacular, particularly on our approach to the park’s airstrip. Our tour was deliberately timed to coincide with the dry season, a time when many of the trees have dropped their leaves, heightening visibility and leaving the landscapes starkly beautiful. This is also a time when the Great Ruaha River and its many smaller tributaries dwindle to shallow, often intermittent “sand rivers,” which, nonetheless, provide natural game corridors and concentration points for birds during a time in which water is at a premium. Bateleur, Ruaha National Park, Sept 2018 (© Kevin J. Zimmer) After touching down at the airstrip, we disembarked to find our trusty drivers, Geitan Ndunguru and Roger Mwengi, each of them longtime friends from our Northern Tanzania tours, waiting for us with their safari vehicles ready for action. The first order of business was to head to the lodge for lunch, but a large, mixed-species coven of Victor Emanuel Nature Tours 2 Southern Tanzania, 2018 vultures could not be ignored, particularly once we discovered the reason for the assemblage—a dead Hippo, no doubt taken down the previous night as it attempted to cross from one river to another, and, the sated Lion that had been gorging itself ever since. -

Reproductive Success and Nestling Growth of the Baywing Parasitized by Screaming and Shiny Cowbirds
Published by the Wilson Ornithological Society VOL. 122, NO. 3 September 2010 PAGES 417–634 The Wilson Journal of Ornithology 122(3):417–431, 2010 REPRODUCTIVE SUCCESS AND NESTLING GROWTH OF THE BAYWING PARASITIZED BY SCREAMING AND SHINY COWBIRDS MARI´AC.DE MA´ RSICO,1,2 BETTINA MAHLER,1 AND JUAN C. REBOREDA1 ABSTRACT.—We studied the breeding biology of the Baywing (Agelaioides badius), a shared host of Screaming (Molothrus rufoaxillaris) and Shiny (M. bonariensis) cowbirds. We monitored 193 nests from December 2002 to March 2007 in the Province of Buenos Aires, Argentina. Baywings used a wide variety of nesting sites, mainly old nests of furnarids. Their breeding season lasted from late November to February and was closely matched by that of Screaming Cowbirds. The breeding season for Shiny Cowbirds started in late September but overlapped that of Baywings. Frequency and intensity of Screaming Cowbird parasitism were 93% and 5 eggs per parasitized nest, while for Shiny Cowbirds they were 16% and 1.4 eggs. Host clutch size was 4.0 6 0.1 eggs and did not vary with time of breeding. Weight at hatching and age of maximum growth were similar for host and Screaming Cowbird nestlings. Shiny Cowbird nestlings had higher weight at hatching and lower age of maximum growth than the other two species. Screaming and Shiny cowbird nestlings had higher growth rates and asymptotic weights than host nestlings. Sex-specific growth curves of Screaming Cowbirds indicated males had higher growth rate and asymptotic weight than females. Only 19% of the nests produced fledglings. Host egg survival, hatching success, and nestling survival were 0.92, 0.88, and 0.94, respectively. -

Assessment of Farming Systems and Cover Configuration Options That Enhance Natural Regulation of Herbivorous Arthropod Abundance in Maize-Fields
Assessment of farming systems and cover configuration options that enhance natural regulation of herbivorous arthropod abundance in maize-fields by Nickson E. Otieno Dissertation presented for the degree of Doctor of Philosophy (Conservation Ecology) at Stellenbosch University Conservation Ecology and Entomology, Faculty of AgriSciences Supervisor: Dr. Shayne M. Jacobs Co-supervisor: Dr. James S. Pryke December 2018 i Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this dissertation electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated) that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. Date: December 2018 Copyright © 2018 Stellenbosch University All rights reserved ii Stellenbosch University https://scholar.sun.ac.za Dissertation format This dissertation is presented as a compilation of 6 chapters. Each chapter is introduced separately and is written, including reference citing and list of bibliography, according to the style of the journal Agriculture, Ecosystems and Environment. The following chapters have already been submitted for publication in journals Chapter 3: Otieno NE, Pryke, JS, Jacobs, SM. The top-down suppression of arthropod herbivory in inter-cropped maize and organic farms: evidence from δ13C and δ15N stable isotope analyses. Journal of Agronomy for Sustainable Development. Chapter 4: Otieno NE, Jacobs, SM, Pryke, JS. Influence of farm structural complexity features and farming systems on insectivorous birds’ contribution to arthropod herbivore regulation in maize fields. -

The Brown-Headed Cowbird: a Model Species for Testing Novel Research 9 Questions in Animal Ecology, Evolution, and Behavior
The Brown-Headed Cowbird: A Model Species for Testing Novel Research 9 Questions in Animal Ecology, Evolution, and Behavior Brian D. Peer, James W. Rivers, Loren Merrill, Scott K. Robinson, and Stephen I. Rothstein Abstract Although the brown-headed cowbird (Molothrus ater) is the most intensively studied brood parasite in the world, much of the research on cowbirds has focused on the negative effects of parasitism. Here, we argue that negative attitudes toward the cowbird have overshadowed opportunities this species provides for advancing our understanding of social behavior, physiology, evolution, and ecology and conservation of birds. Cowbirds are widely distributed, amenable to captive study, and easy to study in areas where they are abundant. Cowbird nestlings must communicate to unrelated host parents, but unlike some parasitic nestlings, they have no specialized adaptations for doing so. In some areas they often share nests with relatives, which may influence the degree of virulence host experience. The generalist strategy of the cowbird can be used to answer questions about the impact of high reproductive output on female cowbirds, maternal allocation of resources into eggs, and the consequences of exposure to a range of pathogens while visiting host nests. Cowbirds and their hosts provide a contrast to cuckoo-host systems because they are at an earlier stage of B. D. Peer (*) Department of Biological Sciences, Western Illinois University, Moline, IL, USA e-mail: [email protected] J. W. Rivers Department of Forest Ecosystems and Society, Oregon State University, Corvallis, OR, USA L. Merrill Illinois Natural History Survey, Prairie Research Institute, University of Illinois, Champaign, IL, USA S.