Goodvin 2018.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

RATES of KARYOTYPIC EVOLUTION in ESTRILDID FINCHES DIFFER BETWEEN 4 ISLAND and CONTINENTAL CLADES 5 6 Daniel M
bioRxiv preprint doi: https://doi.org/10.1101/013987; this version posted January 19, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 1 2 3 RATES OF KARYOTYPIC EVOLUTION IN ESTRILDID FINCHES DIFFER BETWEEN 4 ISLAND AND CONTINENTAL CLADES 5 6 Daniel M. Hooper1,2 and Trevor D. Price3 7 8 1Commitee on Evolutionary Biology, University of Chicago, Chicago, Illinois 60637 9 2 E-mail: [email protected] 10 3Department of Ecology and Evolution, University of Chicago, Chicago, Illinois 60637 11 12 13 Sunday, January 18, 2015 14 15 16 Running head: Chromosome inversions in finches 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 bioRxiv preprint doi: https://doi.org/10.1101/013987; this version posted January 19, 2015. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 2 35 Reasons why chromosomal rearrangements spread to fixation and frequently distinguish 36 related taxa remain poorly understood. We used cytological descriptions of karyotype to 37 identify large pericentric inversions between species of Estrildid finches (family 38 Estrildidae) and a time-dated phylogeny to assess the genomic, geographic, and 39 phylogenetic context of karyotype evolution in this group. -

Tinamiformes – Falconiformes
LIST OF THE 2,008 BIRD SPECIES (WITH SCIENTIFIC AND ENGLISH NAMES) KNOWN FROM THE A.O.U. CHECK-LIST AREA. Notes: "(A)" = accidental/casualin A.O.U. area; "(H)" -- recordedin A.O.U. area only from Hawaii; "(I)" = introducedinto A.O.U. area; "(N)" = has not bred in A.O.U. area but occursregularly as nonbreedingvisitor; "?" precedingname = extinct. TINAMIFORMES TINAMIDAE Tinamus major Great Tinamou. Nothocercusbonapartei Highland Tinamou. Crypturellus soui Little Tinamou. Crypturelluscinnamomeus Thicket Tinamou. Crypturellusboucardi Slaty-breastedTinamou. Crypturellus kerriae Choco Tinamou. GAVIIFORMES GAVIIDAE Gavia stellata Red-throated Loon. Gavia arctica Arctic Loon. Gavia pacifica Pacific Loon. Gavia immer Common Loon. Gavia adamsii Yellow-billed Loon. PODICIPEDIFORMES PODICIPEDIDAE Tachybaptusdominicus Least Grebe. Podilymbuspodiceps Pied-billed Grebe. ?Podilymbusgigas Atitlan Grebe. Podicepsauritus Horned Grebe. Podicepsgrisegena Red-neckedGrebe. Podicepsnigricollis Eared Grebe. Aechmophorusoccidentalis Western Grebe. Aechmophorusclarkii Clark's Grebe. PROCELLARIIFORMES DIOMEDEIDAE Thalassarchechlororhynchos Yellow-nosed Albatross. (A) Thalassarchecauta Shy Albatross.(A) Thalassarchemelanophris Black-browed Albatross. (A) Phoebetriapalpebrata Light-mantled Albatross. (A) Diomedea exulans WanderingAlbatross. (A) Phoebastriaimmutabilis Laysan Albatross. Phoebastrianigripes Black-lootedAlbatross. Phoebastriaalbatrus Short-tailedAlbatross. (N) PROCELLARIIDAE Fulmarus glacialis Northern Fulmar. Pterodroma neglecta KermadecPetrel. (A) Pterodroma -

The Gambia: a Taste of Africa, November 2017
Tropical Birding - Trip Report The Gambia: A Taste of Africa, November 2017 A Tropical Birding “Chilled” SET DEPARTURE tour The Gambia A Taste of Africa Just Six Hours Away From The UK November 2017 TOUR LEADERS: Alan Davies and Iain Campbell Report by Alan Davies Photos by Iain Campbell Egyptian Plover. The main target for most people on the tour www.tropicalbirding.com +1-409-515-9110 [email protected] p.1 Tropical Birding - Trip Report The Gambia: A Taste of Africa, November 2017 Red-throated Bee-eaters We arrived in the capital of The Gambia, Banjul, early evening just as the light was fading. Our flight in from the UK was delayed so no time for any real birding on this first day of our “Chilled Birding Tour”. Our local guide Tijan and our ground crew met us at the airport. We piled into Tijan’s well used minibus as Little Swifts and Yellow-billed Kites flew above us. A short drive took us to our lovely small boutique hotel complete with pool and lovely private gardens, we were going to enjoy staying here. Having settled in we all met up for a pre-dinner drink in the warmth of an African evening. The food was delicious, and we chatted excitedly about the birds that lay ahead on this nine- day trip to The Gambia, the first time in West Africa for all our guests. At first light we were exploring the gardens of the hotel and enjoying the warmth after leaving the chilly UK behind. Both Red-eyed and Laughing Doves were easy to see and a flash of colour announced the arrival of our first Beautiful Sunbird, this tiny gem certainly lived up to its name! A bird flew in landing in a fig tree and again our jaws dropped, a Yellow-crowned Gonolek what a beauty! Shocking red below, black above with a daffodil yellow crown, we were loving Gambian birds already. -

Southwest Pacific Islands: Samoa, Fiji, Vanuatu & New Caledonia Trip Report 11Th to 31St July 2015
Southwest Pacific Islands: Samoa, Fiji, Vanuatu & New Caledonia Trip Report 11th to 31st July 2015 Orange Fruit Dove by K. David Bishop Trip Report - RBT Southwest Pacific Islands 2015 2 Tour Leaders: K. David Bishop and David Hoddinott Trip Report compiled by Tour Leader: K. David Bishop Tour Summary Rockjumper’s inaugural tour of the islands of the Southwest Pacific kicked off in style with dinner at the Stamford Airport Hotel in Sydney, Australia. The following morning we were soon winging our way north and eastwards to the ancient Gondwanaland of New Caledonia. Upon arrival we then drove south along a road more reminiscent of Europe, passing through lush farmlands seemingly devoid of indigenous birds. Happily this was soon rectified; after settling into our Noumea hotel and a delicious luncheon, we set off to explore a small nature reserve established around an important patch of scrub and mangroves. Here we quickly cottoned on to our first endemic, the rather underwhelming Grey-eared Honeyeater, together with Nankeen Night Herons, a migrant Sacred Kingfisher, White-bellied Woodswallow, Fantailed Gerygone and the resident form of Rufous Whistler. As we were to discover throughout this tour, in areas of less than pristine habitat we encountered several Grey-eared Honeyeater by David Hoddinott introduced species including Common Waxbill. And so began a series of early starts which were to typify this tour, though today everyone was up with added alacrity as we were heading to the globally important Rivierre Bleu Reserve and the haunt of the incomparable Kagu. We drove 1.3 hours to the reserve, passing through a stark landscape before arriving at the appointed time to meet my friend Jean-Marc, the reserve’s ornithologist and senior ranger. -
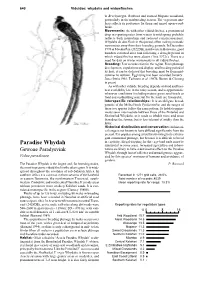
Paradise Whydah Substantial in Some Areas
640 Viduidae: whydahs and widowfinches in Brachystegia, Baikiaea and stunted Mopane woodland, particularly in the nonbreeding season. The vegetation ana- lysis reflects its preference for thorn and mixed open wood- lands. Movements: As with other viduid finches, a pronounced drop in reporting rates from winter to mid-spring probably reflects both nomadism and reduced conspicuousness. Whydahs do also flock in this period, often making nomadic movements away from their breeding grounds. In December 1970 at Mwaku Pan (2022DB), northwestern Botswana, good numbers returned after rain following a drought period in which viduid finches were absent (Tree 1972c). There is a need for data on winter movements in all viduid finches. Breeding: Few records exist for the region. From plumage development, copulations and display, and breeding period of its host, it can be deduced that breeding must be from mid- summer to autumn. Egglaying has been recorded January– June (Irwin 1981; Tarboton et al. 1987b; Brown & Clinning in press). As with other viduids, breeding depends on food and host- nest availability late in the rainy season, and is opportunistic whenever conditions (including mature green seed-heads as food and nestbuilding material for the host) are favourable. Interspecific relationships: It is an obligate brood- parasite of the Melba Finch Pytilia melba, and the ranges of these two species follow the same pattern. Its habitat require- ments seem intermediate between those of the Pintailed and Shafttailed Whydahs, as it tends to inhabit more arid areas than does the former, but is less tolerant of aridity than the latter. Historical distribution and conservation: Its histori- cal range is not known to have differed significantly from the present. -

Flora and Fauna Study
A P P END I X E Flora and Fauna Study November 2010 Environmental I m p a c t S t a t e m e n t – Preliminary R e n e w a b l e P o w e r G e n e r a t i o n a n d R e s o u r c e s R e c o v e r y P l a n t BARRIO CAMBALACHE OF ARECIBO Flora and Fauna Study Preliminary Environmental Impact Statement Renewable Power Generation a n d R e s o u r c e s Recovery Plant BARRIO CAMBALACHE IN ARECIBO CSA ARCHITECTS AND ENGINEERS, LLP 1064 Ponce de León Ave., CSA Plaza Suite 500 San Juan, PR 00907-3740 T 787.641.6800 F 787.641.6850 www.csagroup.com TABLE OF CONTENTS 1.0 EXECUTIVE SUMMARY ........................................................................................................ 1 2.0 INTRODUCTION ................................................................................................................... 3 3.0 GENERAL AREA DESCRIPTION ............................................................................................. 5 3.1. CLIMATE .......................................................................................................................... 6 3.2. HYDROLOGY AND WETLANDS .............................................................................................. 7 3.3. GEOLOGY, TOPOGRAPHY AND SOILS ..................................................................................... 8 3.4. ECOLOGICAL LIFE ZONES ..................................................................................................... 8 3.5. PROTECTED AREAS IN THE REGION ...................................................................................... -

Chewing Lice (Insecta: Phthiraptera) from Estrildid Finches (Aves
Zootaxa 2714: 59–68 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) Chewing lice (Insecta: Phthiraptera) from estrildid finches (Aves: Passeriformes: Estrildidae) and louse-flies (Insecta: Diptera: Hippoboscidae) from birds in Senegal, with descriptions of three new species of the genus Brueelia OLDŘICH SYCHRA1,3, IVAN LITERÁK1, TOMÁŠ NAJER1, MIROSLAV ČAPEK2, PETR KOUBEK2 & PETR PROCHÁZKA2 1Department of Biology and Wildlife Diseases, Faculty of Veterinary Hygiene and Ecology, University of Veterinary and Pharmaceutical Sciences, Palackého 1–3, 612 42 Brno, Czech Republic 2Institute of Vertebrate Biology Academy of Sciences of the Czech Republic, v.v.i., Květná 8, 603 65 Brno, Czech Republic 3Corresponding author. E-mail: [email protected] Abstract Descriptions and illustrations are given for three new species of the genus Brueelia Kéler from estrildid finches (Estrildidae) from Senegal. They and their type hosts are: B. fasciata from Amadina fasciata, B. senegala from Lagonosticta senegala and B. cantans from Euodice cantans. Records of three other louse species of the genus Myrsidea Waterston from estrildid finches and records of louse-flies (Hippoboscidae) from birds in Senegal are also given. Key words: Brueelia, Chewing lice, Estrildidae, Hippoboscidae, louse-flies, Myrsidea, Passeriformes, Phthiraptera, Senegal Introduction The family Estrildidae comprising waxbills, munias and allies represents a relatively large family of passerine birds. About 141 species of these gregarious and often colonial seed-eaters are distributed in the Old World tropics and Australasia, of which 23 occur in Senegal (Fry & Keith 2004, Lepage 2009, according to taxonomy in Clements 2007). Data concerning records of chewing lice from Senegalese Estrildidae are scarce. -

Senegal and Gambia
BIRDING AFRICA THE AFRICA SPECIALISTS Senegal and Gambia 2019 Tour Report Vinaceous Black-faced Firefi nch Text by tour leader Michael Mills Photos by Gus Mills SUMMARY ESSENTIAL DETAILS Our first trip to Senegal and Gambia was highly successful and netted a Dates 16 Jan: Full day in the Kedougou area seeing Mali good selection of localised and rarely-seen specials. For this private trip we Firefinch.. ran a flexible itinerary to target a small selection of tricky species. In Senegal 11-23 January 2019 17 Jan: Early departure from Kedougou. Lunch Savile's Bustard was the last African bustard for the entire party, and was at Wassadou Camp. Evening boat trip on Gambia Birding Africa Tour Report Tour Africa Birding seen well both at the Marigots and in the Kaolack area. Other Senegalese Leaders River with Adamawa Turtle Dove and Egyptian Report Tour Africa Birding Plover.. highlights included Western Red-billed Hornbill, Sahel Paradise Whydah Michael Mills assisted by Solomon Jallow in full breeding plumage, Little Grey Woodpecker, Mali Firefinch, Egyptian 18 Jan: Morning at Wassadou Camp, before driving to Gambia. Afternoon around Bansang with Plover and African Finfoot. Large numbers of waterbirds at Djoudj and the Participants Exclamatory Paradise Whydah in full plumage. Marigots were memorable too. Julian Francis and Gus Mills 19 Jan: Early departure from Bansang, driving to Tendaba for lunch via north bank. Afternoon at Tendaba seeing Bronze-winged Courser. Itinerary 20 Jan: Early morning on the Bateling Track seeing 11 Jan: Dakar to St Louis. Afternoon at the Marigots, Yellow Penduline Tit, White-fronted Black Chat, hearing Savile's Bustard. -

GHANA MEGA Rockfowl & Upper Guinea Specials Th St 29 November to 21 December 2011 (23 Days)
GHANA MEGA Rockfowl & Upper Guinea Specials th st 29 November to 21 December 2011 (23 days) White-necked Rockfowl by Adam Riley Trip Report compiled by Tour Leader David Hoddinott RBT Ghana Mega Trip Report December 2011 2 Trip Summary Our record breaking trip total of 505 species in 23 days reflects the immense birding potential of this fabulous African nation. Whilst the focus of the tour was certainly the rich assemblage of Upper Guinea specialties, we did not neglect the interesting diversity of mammals. Participants were treated to an astonishing 9 Upper Guinea endemics and an array of near-endemics and rare, elusive, localized and stunning species. These included the secretive and rarely seen White-breasted Guineafowl, Ahanta Francolin, Hartlaub’s Duck, Black Stork, mantling Black Heron, Dwarf Bittern, Bat Hawk, Beaudouin’s Snake Eagle, Congo Serpent Eagle, the scarce Long-tailed Hawk, splendid Fox Kestrel, African Finfoot, Nkulengu Rail, African Crake, Forbes’s Plover, a vagrant American Golden Plover, the mesmerising Egyptian Plover, vagrant Buff-breasted Sandpiper, Four-banded Sandgrouse, Black-collared Lovebird, Great Blue Turaco, Black-throated Coucal, accipiter like Thick- billed and splendid Yellow-throated Cuckoos, Olive and Dusky Long-tailed Cuckoos (amongst 16 cuckoo species!), Fraser’s and Akun Eagle-Owls, Rufous Fishing Owl, Red-chested Owlet, Black- shouldered, Plain and Standard-winged Nightjars, Black Spinetail, Bates’s Swift, Narina Trogon, Blue-bellied Roller, Chocolate-backed and White-bellied Kingfishers, Blue-moustached, -

Federal Register/Vol. 85, No. 74/Thursday, April 16, 2020/Notices
21262 Federal Register / Vol. 85, No. 74 / Thursday, April 16, 2020 / Notices acquisition were not included in the 5275 Leesburg Pike, Falls Church, VA Comment (1): We received one calculation for TDC, the TDC limit would not 22041–3803; (703) 358–2376. comment from the Western Energy have exceeded amongst other items. SUPPLEMENTARY INFORMATION: Alliance, which requested that we Contact: Robert E. Mulderig, Deputy include European starling (Sturnus Assistant Secretary, Office of Public Housing What is the purpose of this notice? vulgaris) and house sparrow (Passer Investments, Office of Public and Indian Housing, Department of Housing and Urban The purpose of this notice is to domesticus) on the list of bird species Development, 451 Seventh Street SW, Room provide the public an updated list of not protected by the MBTA. 4130, Washington, DC 20410, telephone (202) ‘‘all nonnative, human-introduced bird Response: The draft list of nonnative, 402–4780. species to which the Migratory Bird human-introduced species was [FR Doc. 2020–08052 Filed 4–15–20; 8:45 am]‘ Treaty Act (16 U.S.C. 703 et seq.) does restricted to species belonging to biological families of migratory birds BILLING CODE 4210–67–P not apply,’’ as described in the MBTRA of 2004 (Division E, Title I, Sec. 143 of covered under any of the migratory bird the Consolidated Appropriations Act, treaties with Great Britain (for Canada), Mexico, Russia, or Japan. We excluded DEPARTMENT OF THE INTERIOR 2005; Pub. L. 108–447). The MBTRA states that ‘‘[a]s necessary, the Secretary species not occurring in biological Fish and Wildlife Service may update and publish the list of families included in the treaties from species exempted from protection of the the draft list. -

Manyoni Private Game Reserve (Previously Zululand Rhino Reserve)
Manyoni Private Game Reserve (Previously Zululand Rhino Reserve) Gorgeous Bushshrike by Adam Riley BIRD LIST Prepared by Adam Riley [email protected] • www.rockjumperbirding.