1 Convergent Evolution of Reduced Eggshell Conductance in Avian
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bird Checklists of the World Country Or Region: Ghana
Avibase Page 1of 24 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Ghana 2 Number of species: 773 3 Number of endemics: 0 4 Number of breeding endemics: 0 5 Number of globally threatened species: 26 6 Number of extinct species: 0 7 Number of introduced species: 1 8 Date last reviewed: 2019-11-10 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Ghana. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=gh [26/09/2021]. Make your observations count! Submit your data to ebird. -

South Africa Mega Birding Tour I 6Th to 30Th January 2018 (25 Days) Trip Report
South Africa Mega Birding Tour I 6th to 30th January 2018 (25 days) Trip Report Aardvark by Mike Bacon Trip report compiled by Tour Leader: Wayne Jones Rockjumper Birding Tours View more tours to South Africa Trip Report – RBT South Africa - Mega I 2018 2 Tour Summary The beauty of South Africa lies in its richness of habitats, from the coastal forests in the east, through subalpine mountain ranges and the arid Karoo to fynbos in the south. We explored all of these and more during our 25-day adventure across the country. Highlights were many and included Orange River Francolin, thousands of Cape Gannets, multiple Secretarybirds, stunning Knysna Turaco, Ground Woodpecker, Botha’s Lark, Bush Blackcap, Cape Parrot, Aardvark, Aardwolf, Caracal, Oribi and Giant Bullfrog, along with spectacular scenery, great food and excellent accommodation throughout. ___________________________________________________________________________________ Despite havoc-wreaking weather that delayed flights on the other side of the world, everyone managed to arrive (just!) in South Africa for the start of our keenly-awaited tour. We began our 25-day cross-country exploration with a drive along Zaagkuildrift Road. This unassuming stretch of dirt road is well-known in local birding circles and can offer up a wide range of species thanks to its variety of habitats – which include open grassland, acacia woodland, wetlands and a seasonal floodplain. After locating a handsome male Northern Black Korhaan and African Wattled Lapwings, a Northern Black Korhaan by Glen Valentine -

Zambia and Zimbabwe 28 �Ovember – 6 December 2009
Zambia and Zimbabwe 28 ovember – 6 December 2009 Guide: Josh Engel A Tropical Birding Custom Tour All photos taken by the guide on this tour. The Smoke that Thunders: looking down one end of the mile-long Victoria Falls. ITRODUCTIO We began this tour by seeing one of Africa’s most beautiful and sought after birds: African Pitta . After that, the rest was just details. But not really, considering we tacked on 260 more birds and loads of great mammals. We saw Zambia’s only endemic bird, Chaplin’s Barbet , as well as a number of miombo and broad-leaf specialties, including Miombo Rock-Thrush, Racket-tailed Roller, Southern Hyliota, Miombo Pied Barbet, Miombo Glossy Starling, Bradfield’s Hornbill, Pennant-winged ightjar, and Three-banded Courser. With the onset of the rainy season just before the tour, the entire area was beautifully green and was inundated with migrants, so we were able to rack up a great list of cuckoos and other migrants, including incredible looks at a male Kurrichane Buttonquail . Yet the Zambezi had not begun to rise, so Rock Pratincole still populated the river’s rocks, African Skimmer its sandbars, and Lesser Jacana and Allen’s Gallinule its grassy margins. Mammals are always a highlight of any Africa tour: this trip’s undoubted star was a leopard , while a very cooperative serval was also superb. Victoria Falls was incredible, as usual. We had no problems in Zimbabwe whatsoever, and our lodge there on the shores of the Zambezi River was absolutely stunning. The weather was perfect throughout the tour, with clouds often keeping the temperature down and occasional rains keeping bird activity high. -
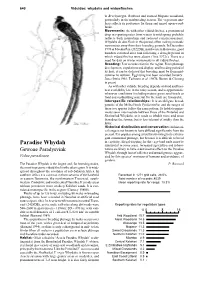
Paradise Whydah Substantial in Some Areas
640 Viduidae: whydahs and widowfinches in Brachystegia, Baikiaea and stunted Mopane woodland, particularly in the nonbreeding season. The vegetation ana- lysis reflects its preference for thorn and mixed open wood- lands. Movements: As with other viduid finches, a pronounced drop in reporting rates from winter to mid-spring probably reflects both nomadism and reduced conspicuousness. Whydahs do also flock in this period, often making nomadic movements away from their breeding grounds. In December 1970 at Mwaku Pan (2022DB), northwestern Botswana, good numbers returned after rain following a drought period in which viduid finches were absent (Tree 1972c). There is a need for data on winter movements in all viduid finches. Breeding: Few records exist for the region. From plumage development, copulations and display, and breeding period of its host, it can be deduced that breeding must be from mid- summer to autumn. Egglaying has been recorded January– June (Irwin 1981; Tarboton et al. 1987b; Brown & Clinning in press). As with other viduids, breeding depends on food and host- nest availability late in the rainy season, and is opportunistic whenever conditions (including mature green seed-heads as food and nestbuilding material for the host) are favourable. Interspecific relationships: It is an obligate brood- parasite of the Melba Finch Pytilia melba, and the ranges of these two species follow the same pattern. Its habitat require- ments seem intermediate between those of the Pintailed and Shafttailed Whydahs, as it tends to inhabit more arid areas than does the former, but is less tolerant of aridity than the latter. Historical distribution and conservation: Its histori- cal range is not known to have differed significantly from the present. -

Notes on Honeyguides in Southeast Cape Province, South Africa
52 SK•AD,Honeyguides ofSoutheast CapeProvince t[AukJan. NOTES ON HONEYGUIDES IN SOUTHEAST CAPE PROVINCE, SOUTH AFRICA BY C. J. SKEAD Now that I am no longer living on a farm it seemsadvisable to collate what little data I have accumulatedon honeyguidesand add it to the scanty knowledgewe have of these birds, in this instance the LesserHoneyguide, Indicator minor minor, the Greater Honeyguide, Indicator indicator, and Wahlberg's Sharp-billed Honeyguide, Pro- dotiscusregulus regulus. The farm "Gameston" where these records were taken lies in the grass-and bush-coveredupper reachesof the Kariega River, 15 miles southwestof Grahamstown, Albany district, Southeast Cape Province, South Africa. This veld-type, known as "bontebosveld," spreads over a large part of the southeastcape. Although I have known the birds for many years the written recordscover a period of about five years. (1) THE LESSERHONEYGUIDE F.colog3.--This speciesprefers the scrub bushyeld and more open acaciaveld, sitting amongthe inner branchesof the trees and bushes but makingconspicuous flights in the openfrom one patch of bushto another. Status.--I have recordedthem in January, February, March, July, August,September, and December. This suggeststhey are resident and not migratory. They are very restlessbirds being seldomseen for more than an hour or so and, in my experience,usually one at a time, even when they parasitized a nest of Black-collared Barbers, Lybius torquatus,in our garden. But from July to September, 1949, two often consortedtogether, attracted, I think, by combs of honey protrudingfrom the gardenbee-hives. There were no barbetsin the garden then. Field Characters.--Thereare no striking features; their dull colora- tion is lightly offset by the golden sheen seen on the back when the light is good. -

Manyoni Private Game Reserve (Previously Zululand Rhino Reserve)
Manyoni Private Game Reserve (Previously Zululand Rhino Reserve) Gorgeous Bushshrike by Adam Riley BIRD LIST Prepared by Adam Riley [email protected] • www.rockjumperbirding. -

Zimbabwe Zambia Malawi Species Checklist Africa Vegetation Map
ZIMBABWE ZAMBIA MALAWI SPECIES CHECKLIST AFRICA VEGETATION MAP BIOMES DeserT (Namib; Sahara; Danakil) Semi-deserT (Karoo; Sahel; Chalbi) Arid SAvannah (Kalahari; Masai Steppe; Ogaden) Grassland (Highveld; Abyssinian) SEYCHELLES Mediterranean SCruB / Fynbos East AFrican Coastal FOrest & SCruB DrY Woodland (including Mopane) Moist woodland (including Miombo) Tropical Rainforest (Congo Basin; upper Guinea) AFrO-Montane FOrest & Grassland (Drakensberg; Nyika; Albertine rift; Abyssinian Highlands) Granitic Indian Ocean IslandS (Seychelles) INTRODUCTION The idea of this booklet is to enable you, as a Wilderness guest, to keep a detailed record of the mammals, birds, reptiles and amphibians that you observe during your travels. It also serves as a compact record of your African journey for future reference that hopefully sparks interest in other wildlife spheres when you return home or when travelling elsewhere on our fragile planet. Although always exciting to see, especially for the first-time Africa visitor, once you move beyond the cliché of the ‘Big Five’ you will soon realise that our wilderness areas offer much more than certain flagship animal species. Africa’s large mammals are certainly a big attraction that one never tires of, but it’s often the smaller mammals, diverse birdlife and incredible reptiles that draw one back again and again for another unparalleled visit. Seeing a breeding herd of elephant for instance will always be special but there is a certain thrill in seeing a Lichtenstein’s hartebeest, cheetah or a Lilian’s lovebird – to name but a few. As a globally discerning traveller, look beyond the obvious, and challenge yourself to learn as much about all wildlife aspects and the ecosystems through which you will travel on your safari. -

A Plant Ecological Study and Management Plan for Mogale's Gate Biodiversity Centre, Gauteng
A PLANT ECOLOGICAL STUDY AND MANAGEMENT PLAN FOR MOGALE’S GATE BIODIVERSITY CENTRE, GAUTENG By Alistair Sean Tuckett submitted in accordance with the requirements for the degree of MASTER OF SCIENCE in the subject ENVIRONMENTAL MANAGEMENT at the UNIVERSITY OF SOUTH AFRICA SUPERVISOR: PROF. L.R. BROWN DECEMBER 2013 “Like winds and sunsets, wild things were taken for granted until progress began to do away with them. Now we face the question whether a still higher 'standard of living' is worth its cost in things natural, wild and free. For us of the minority, the opportunity to see geese is more important that television.” Aldo Leopold 2 Abstract The Mogale’s Gate Biodiversity Centre is a 3 060 ha reserve located within the Gauteng province. The area comprises grassland with woodland patches in valleys and lower-lying areas. To develop a scientifically based management plan a detailed vegetation study was undertaken to identify and describe the different ecosystems present. From a TWINSPAN classification twelve plant communities, which can be grouped into nine major communities, were identified. A classification and description of the plant communities, as well as, a management plan are presented. The area comprises 80% grassland and 20% woodland with 109 different plant families. The centre has a grazing capacity of 5.7 ha/LSU with a moderate to good veld condition. From the results of this study it is clear that the area makes a significant contribution towards carbon storage with a total of 0.520 tC/ha/yr stored in all the plant communities. KEYWORDS Mogale’s Gate Biodiversity Centre, Braun-Blanquet, TWINSPAN, JUICE, GRAZE, floristic composition, carbon storage 3 Declaration I, Alistair Sean Tuckett, declare that “A PLANT ECOLOGICAL STUDY AND MANAGEMENT PLAN FOR MOGALE’S GATE BIODIVERSITY CENTRE, GAUTENG” is my own work and that all sources that I have used or quoted have been indicated and acknowledged by means of complete references. -

The Gambia in Style
The Gambia in Style Naturetrek Tour Report 7 - 14 December 2018 Broad-billed Roller Blue-breasted Kingfisher Western Red Colobus Little Bee-eater Report compiled by Duncan McNiven Images courtesy of Debbie Pain Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ UK T: +44 (0)1962 733051 E: [email protected] W: www.naturetrek.co.uk Tour Report The Gambia in Style Tour participants: Duncan McNiven and Debbie Pain (leaders) plus local guides and 12 Naturetrek clients Day 1 Friday 7th December London Gatwick to Mandina Lodge We all met up at Gatwick Airport for our mid-morning Titan Airways flight to Banjul, the capital of Gambia. Our flight was slightly delayed due to inclement weather but soon enough we were on our way south, leaving behind the mountains and plains of southern Europe and crossing the vast aridness of the Sahara Desert. Eventually, the landscape below us became greener and lusher as we crossed in to Senegal and entered the Guinea Savannah biome. By the time we touched down in Banjul it was already early evening and the light was beginning to wane. Duncan and Debbie were there to meet and greet us and help with acquiring the small amount of local currency we would require for our holiday and in no time at all we were aboard our bus for our short journey to Mandina Lodge. Less than an hour later we were sat under the twinkling fairy lights of the Mandina reception area whilst our gracious host Linda welcomed us and gave us the briefest of briefings before we were all shown to our luxurious lodges nestled amongst the mangroves and forest trees lining the river. -

Project Name
SYRAH RESOURCES GRAPHITE PROJECT, CABO DELGADO, MOZAMBIQUE TERRESTRIAL FAUNAL IMPACT ASSESSMENT Prepared by: Prepared for: Syrah Resources Limited Coastal and Environmental Services Mozambique, Limitada 356 Collins Street Rua da Frente de Libertação de Melbourne Moçambique, Nº 324 3000 Maputo- Moçambique Australia Tel: (+258) 21 243500 • Fax: (+258) 21 243550 Website: www.cesnet.co.za December 2013 Syrah Final Faunal Impact Assessment – December 2013 AUTHOR Bill Branch, Terrestrial Vertebrate Faunal Consultant Bill Branch obtained B.Sc. and Ph.D. degrees at Southampton University, UK. He was employed for 31 years as the herpetologist at the Port Elizabeth Museum, and now retired holds the honorary post of Curator Emeritus. He has published over 260 scientific articles, as well as numerous popular articles and books. The latter include the Red Data Book for endangered South African reptiles and amphibians (1988), and co-editing its most recent upgrade – the Atlas and Red Data Book of the Reptiles of South Africa, Lesotho and Swaziland (2013). He has also published guides to the reptiles of both Southern and Eastern Africa. He has chaired the IUCN SSC African Reptile Group. He has served as an Honorary Research Professor at the University of Witwatersrand (Johannesburg), and has recently been appointed as a Research Associate at the Nelson Mandela Metropolitan University, Port Elizabeth. His research concentrates on the taxonomy, biogeography and conservation of African reptiles, and he has described over 30 new species and many other higher taxa. He has extensive field work experience, having worked in over 16 African countries, including Gabon, Ivory Coast, DRC, Zambia, Mozambique, Malawi, Madagascar, Namibia, Angola and Tanzania. -

Adult Brood Parasites Feeding Nestlings and Fledglings of Their Own Species: a Review
J. Field Ornithol., 69(3):364-375 ADULT BROOD PARASITES FEEDING NESTLINGS AND FLEDGLINGS OF THEIR OWN SPECIES: A REVIEW JANICEC. LORENZANAAND SPENCER G. SEALY Departmentof Zoology Universityof Manitoba Winnipeg,Manitoba R3T 2N2 Canada Abstract.--We summarized 40 reports of nine speciesof brood parasitesfeeding young of their own species.These observationssuggest that the propensityto provisionyoung hasnot been lost entirely in brood parasitesdespite the belief that brood parasiticadults abandon their offspringat the time of laying.The hypothesisthat speciesthat participatein courtship feeding are more likely to provisionyoung was not supported:provisioning of young has been observedin two speciesof brood parasitesthat do not courtshipfeed. The function of this provisioningis unknown, but we suggestit may be: (1) a non-adaptivevestigial behavior or (2) an adaptation to ensure adequatecare of parasiticyoung. The former is more likely the case.Further studiesare required to determinewhether parasiticadults commonly feed their genetic offspring. ADULTOS DE AVES PARAS•TICASALIMENTANDO PICHONES Y VOLANTONES DE SU PROPIA ESPECIE: UNA REVISION Sinopsis.--Resumimos40 informes de nueve especiesde avesparasiticas que alimenaron a pichonesde su propia especie.Las observacionessugieren que la propensividadde alimentar a los pichonesno ha sido totalmente perdida en las avesparasiticas, no empecea la creencia de que los parasiticosabandonan su progenie al momento de poner los huevos.La hipttesis de que las especiesque participan en cortejo de alimentacitn, son milspropensas a alimentar los pichonesno tuvo apoyo.Las observacionesde alimentacitn a pichonesse han hecho en dos especiesparasiticas cuyo cortejo no incluye la alimentacitn de la pareja. La funcitn de proveer alimento se desconoce.No obstante,sugerimos que pueda ser: 1) una conducta vestigialno adaptativa,o 2) una adaptacitn parc asegurarel cuidado adecuadode los pi- chonesparasiticos. -

Brain Size and Morphology of the Brood-Parasitic and Cerophagous Honeyguides (Aves: Piciformes)
Original Paper Brain Behav Evol Received: August 2, 2012 DOI: 10.1159/000348834 Returned for revision: September 9, 2012 Accepted after second revision: February 10, 2013 Published online: April 24, 2013 Brain Size and Morphology of the Brood-Parasitic and Cerophagous Honeyguides (Aves: Piciformes) e b a, c Jeremy R. Corfield Tim R. Birkhead Claire N. Spottiswoode e d f Andrew N. Iwaniuk Neeltje J. Boogert Cristian Gutiérrez-Ibáñez g f g Sarah E. Overington Douglas R. Wylie Louis Lefebvre a DST/NRF Center of Excellence, Percy FitzPatrick Institute, University of Cape Town, Cape Town , South Africa; b c Department of Animal and Plant Sciences, University of Sheffield, Sheffield , Department of Zoology, d University of Cambridge, Cambridge , and School of Biology, University of St. Andrews, St. Andrews , UK; e f Department of Neuroscience, University of Lethbridge, Lethbridge, Alta. , Center for Neuroscience, g University of Alberta, Edmonton, Alta. , and Department of Biology, McGill University, Montreal, Que. , Canada Key Words fers greatly between honeyguides and woodpeckers. The Brain size · Brood parasitism · Hippocampus · Piciformes · relatively smaller brains of the honeyguides may be a conse- Volumetrics quence of brood parasitism and cerophagy (‘wax eating’), both of which place energetic constraints on brain develop- ment and maintenance. The inconclusive results of our anal- Abstract yses of relative HF volume highlight some of the problems Honeyguides (Indicatoridae, Piciformes) are unique among associated with comparative studies of the HF that require birds in several respects. All subsist primarily on wax, are further study. Copyright © 2013 S. Karger AG, Basel obligatory brood parasites and one species engages in ‘guid- ing’ behavior in which it leads human honey hunters to bees’ nests.