The Fetal Circulation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CCM2 and CCM3 Proteins Contribute to Vasculogenesis and Angiogenesis in Human Placenta
Histol Histopathol (2009) 24: 1287-1294 Histology and http://www.hh.um.es Histopathology Cellular and Molecular Biology CCM2 and CCM3 proteins contribute to vasculogenesis and angiogenesis in human placenta Gamze Tanriover1, Yasemin Seval1, Leyla Sati1, Murat Gunel2 and Necdet Demir1 1Department of Histology and Embryology, Akdeniz University, School of Medicine, Antalya, Turkey and 2 Department of Neurosurgery, Yale University, School of Medicine, New Haven, CT, USA Summary. Placenta as an ideal model to study Introduction angiogenic mechanisms have been established in previous studies. There are two processes, The placenta is a multifaceted organ that plays a vasculogenesis and angiogenesis, involved in blood critical role in maintaining and protecting the developing vessel formation during placental development. fetus. Normal development and function of the placenta Therefore, blood vessel formation is a crucial issue that requires extensive vasculogenesis and subsequent might cause vascular malformations. One of the vascular angiogenesis, in both maternal and fetal tissues. malformations is cerebral cavernous malformation Vasculogenesis is the formation of the primitive vascular (CCM) in the central nervous system, consisting of network de novo from progenitor cells, and angiogenesis endothelium-lined vascular channels without intervening is identified as the extension of blood vessels from normal brain parenchyma. Three CCM loci have been preexisting vascular structures (Demir et al., 1989, 2006; mapped as Ccm1, Ccm2, Ccm3 genes in CCM. In order Geva et al., 2002; Charnock-Jones et al., 2004). Many to investigate whether CCM proteins participate in blood factors, such as vascular endothelial growth factor vessel formation, we report here the expression patterns (VEGF), angiopoietins (Angpt-1 and -2) and their of CCM2 and CCM3 in developing and term human receptors are involved in the molecular regulation of placenta by means of immunohistochemistry and these diverse developmental steps. -

Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power
DSJUOG Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power Doppler Technology REVIEW ARTICLE Fetal Brain Vascularity Visualized by Conventional 2D and 3D Power Doppler Technology 1Ritsuko K Pooh, 2Asim Kurjak 1CRIFM Clinical Research Institute of Fetal Medicine PMC, Osaka, Japan 2Department of Obstetrics and Gynecology, University of Zagreb, School of Medicine, Zagreb, Croatia Correspondence: Ritsuko K Pooh, CRIFM Clinical Research Institute of Fetal Medicine PMC 7-3-7, Uehommachi, Tennoji Osaka #543-0001, Japan, Phone: +81-6-6775-8111, Fax: +81-6-6775-8122, e-mail: [email protected] Abstract Significant advances have been made in accurate and reliable visualization of the cerebral circulation in normal and abnormal pregnancies. They provided the non-invasive studies of fetal cerebral angiogenesis and further development that filled some of the gaps made by neuroanatomical studies alone. The first breakthrough in the assessment of fetal circulation was development of Doppler system with purpose to obtain velocity waveforms. Continuing technical advances in Doppler ultrasound equipment, especially highly sensitive color flow imagining techniques have made it possible to study smaller anatomical parts of fetal circulation system including cerebral vascularization. Before examination of brain vascularity, anatomical vascular structure and development on the different appearance at each gestational age should be remembered as the basic knowledge. Since the development of the embryo is rapid and significant changes occur during even one week it is important to specify the stage of the embryo or fetus both by age (postmenstrual weeks and days) and by size (crownrump length (CRL) and biparietal diameter (BPD). Introduction of three-dimensional (3D) sonography and 3D power Doppler techniques have enabled visualization of intracranial vessels. -

Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease
Journal of Cardiovascular Development and Disease Review Fetal Blood Flow and Genetic Mutations in Conotruncal Congenital Heart Disease Laura A. Dyer 1 and Sandra Rugonyi 2,* 1 Department of Biology, University of Portland, Portland, OR 97203, USA; [email protected] 2 Department of Biomedical Engineering, Oregon Health & Science University, Portland, OR 97239, USA * Correspondence: [email protected] Abstract: In congenital heart disease, the presence of structural defects affects blood flow in the heart and circulation. However, because the fetal circulation bypasses the lungs, fetuses with cyanotic heart defects can survive in utero but need prompt intervention to survive after birth. Tetralogy of Fallot and persistent truncus arteriosus are two of the most significant conotruncal heart defects. In both defects, blood access to the lungs is restricted or non-existent, and babies with these critical conditions need intervention right after birth. While there are known genetic mutations that lead to these critical heart defects, early perturbations in blood flow can independently lead to critical heart defects. In this paper, we start by comparing the fetal circulation with the neonatal and adult circulation, and reviewing how altered fetal blood flow can be used as a diagnostic tool to plan interventions. We then look at known factors that lead to tetralogy of Fallot and persistent truncus arteriosus: namely early perturbations in blood flow and mutations within VEGF-related pathways. The interplay between physical and genetic factors means that any one alteration can cause significant disruptions during development and underscore our need to better understand the effects of both blood flow and flow-responsive genes. -
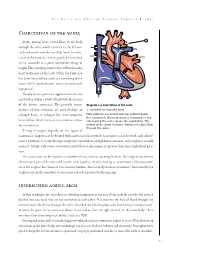
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Patent Ductus Arteriosus About This Factsheet the Normal Heart
Understanding your child’s heart Patent ductus arteriosus About this factsheet The normal heart This factsheet is for parents of babies and children who The heart is a muscular pump which pumps blood through the have patent ductus arteriosus (PDA), which is also known as body and lungs. There are four chambers in the heart. The two persistent arterial duct. upper ones are called the right atrium and left atrium. These are separated by a wall called the atrial septum. The two lower It explains: chambers are called the right and left ventricles, and are separated • what patent ductus arteriosus is and how it is diagnosed by a wall called the ventricular septum. • how patent ductus arteriosus is treated • the benefits and risks of treatments. On each side of the heart, blood passes from the atrium, through a heart valve – the tricuspid valve on the right, and the mitral valve This factsheet does not replace the advice that doctors or on the left – into the ventricle. The ventricles are the main pumping nurses may give you, but it should help you to understand chambers of the heart. Each ventricle pumps blood out into an artery. what they tell you. The right ventricle pumps blood – blue in the illustration – into the pulmonary artery (the blood vessel that takes blood to the lungs). The left ventricle pumps blood – red in the illustration – into the aorta (the blood vessel that takes blood to the rest of the body). Blood flows from the right side of the heart, through the pulmonary valve into the pulmonary artery, and then to the lungs where it picks up oxygen. -

Development of HEART 4-VEINS
Development of brachiocephalic veins 1. Right brachiocephalic vein is formed by cranial part of right anterior cardinal vein and 2. Left brachiocephalic is formed by cranial part of left anterior cardinal vein and the interant.cardinal anastomosis. Development of superior vena cava 1. The part up to the opening of vena azygos develops from caudal part of right ant.cardinal vein and 2. The part below the opening (intrapericardial part) is formed by the right common cardinal vein. Development of azygos and hemiazygos veins A. 1. Vena azygos develops from right azygos line vein and 2. The arch of vena azygos is formed by the cranial end of right postcardinal vein. B. Hemiazygos veins are formed by the left azygos line vein. Development of Inferior vena cava Inferior vena cava is formed, from below upwards by: 1. Begins by the union of the two common iliac veins (postcardinal veins), 2. Right supracardinal, 3. Right supra-subcardinal anastomosis, 4. Right subcardinal, 5. New formation (hepatic segment) and 6. Hepatocardiac channel (terminal part of right vitelline vein). Congenital anomalies • Double inferior vena cava • Absence • Left SVC • Double SVC DEVELOPMENT OF PORTAL VEIN 1. The portal vein is formed behind the neck of pancreas by the union of superior mesentric and splenic vein to the left vitelline vein. 2. The part of the portal vein which is behind the Ist part of duodenum is formed by middle dorsal transverse anastomosis. 3. Part of portal vein which is in the free margin of lesser omentum is formed by cranial or distal part of right vitelline vein. -

The Placenta
The placenta Learning module Developed by Carolyn Hammer Edited by Fabien Giroux Diagrams By Dr Julien Yockell Lelievre where indicated The placenta – Learning module Table of content 1) Introduction……………………………………………………………………….3 2) Anatomy and Physiology………………………………………………………..6 3) Roles and Functions……………………………………………………………17 4) Development and formation…………………………………..…………….…27 5) What happens after birth……………………………………………….……...34 6) What happens when things go wrong.……………………………………….36 7) Interesting facts about pregnancy………………….…………………………46 2 The placenta – Learning module Introduction 3 The placenta – Learning module What is the placenta? •The placenta is a “vascular (supplied with blood vessels) organ in most mammals that unites the fetus to the uterus of the mother. It mediates the metabolic exchanges of the developing individual through an intimate association of embryonic tissues and of certain uterine tissues, serving the functions of nutrition, respiration, and excretion.” (Online Britannica encyclopaedia) •As the fetus is in full development, it requires a certain amount of gases and nutrients to help support its growth. Because the fetus is unable to do so on its own, the placenta provides these gases and nutrients throughout pregnancy. http://health.allrefer.com/health/plac enta-abruptio-placenta.html 4 The placenta – Learning module What are the main roles of the placenta? •The placenta provides the connection between fetus and mother in order to help carry out many different functions that the growing baby is incapable to do so alone. During pregnancy, the placenta has 6 main roles to maintain good health and a good environment for the growing child: •Respiration •Nutrition •Excretion •Protection •Endocrine •Immunity 5 The placenta – Learning module Anatomy and physiology 6 The placenta – Learning module Structure •A placenta is an organ of round or oval shape that is relatively flat. -

The Structural Heterogeneity of Chorial Villi Phenotype Determined by Angiogenesis
Rom J Leg Med [19] 197-210 [2011] DOI: 10.4323/rjlm.2011.197 © 2011 Romanian Society of Legal Medicine The structural heterogeneity of chorial villi phenotype determined by angiogenesis. Implications in legal pathology Gheorghe S. Dragoi*, Petru Razvan Melinte, Daniel Zimta, Mohab El Din Mohamed _________________________________________________________________________________________ Abstract: Micro anatomic phenotype of chorial villi can be achieved only by means of a rigurous evaluation of its structural elements: trophoblast, vascular and mesenchyme. The authors proposed themselves to study the reciprocal relations between syncytiotrophoblast, fetal sinusoid capillaries and argentic collagen fiber fascicles inside chorial villi depending on angionesis process. The research was carried out on human biologic material using placenta fragments during 28-37 weeks of gestation. The authors consider that the collagen IV stereo distribution inside the vascular pedicle of the terminal villi, contributes to the stability, biodynamic and biokinematics of villi phenotype that is determined by branching or non branching angiogenesis. The personal results have a great value for stating the variability limits of terminal villi phenotype in ortology as well as in general or forensic pathology. Key Words: terminal villi, angiogenesis, collagen IV, villi phenotype he chorial villi phenotype is determined either by vasculogenesis either by angiogenesis. It Tis considered that until the end of gestation, the blood capillaries network reaches 550 km in length and 15 m2 in surface (Burton and Jauniaux, 1995) [7]. Angiogenesis plays an important role in formation and remodeling of blood vessels inside human placenta terminal villi. In the second half of gestation there is a growth acceleration and an increase in number for mature intermediate and terminal villi and for sinusoid blood capillaries (Mayhew, 2002) [22]. -
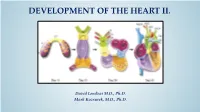
Development of Right Ventricle
DEVELOPMENT OF THE HEART II. David Lendvai M.D., Ph.D. Mark Kozsurek, M.D., Ph.D. • Septation of the common atrioventricular (AV) orifice. • Formation of the interatrial septum. • Formation of the muscular interventricular septum. • Appearance of the membranous interventricular septum and the spiral aorticopulmonary septum. right left septum primum septum primum septum primum septum primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum foramen primum foramen primum septum primum foramen secundum septum primum foramen secundum foramen primum foramen primum septum primum septum primum foramen secundum foramen secundum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum foramen ovale foramen ovale septum primum septum primum septum secundum septum secundum foramen secundum septum primum foramen ovale foramen ovale septum primum SUMMARY • The septation of the common atrium starts with the appearance of the crescent-shaped septum primum. The opening of this septum, the foramen primum, becomes progressively smaller. • Before the foramen primum completly closes, postero-superiorly several small openings appear on the septum primum. These perforations coalesce later and form the foramen secundum. • On the right side of the septum primum a new septum, the septum secundum, starts to grow. The orifice of the septum secundum is the foramen ovale. • Finally two crescent-like, incomplete, partially overlapping septa exist with one hole on each. Septum secundum is more rigid and the septum primum on its left side acts as a valve letting the blood flow exclusively from the right to the left. -

Fetal Circulation
The Fetal Circulation Dr. S. Mathieu, Specialist Registrar in Anaesthesia Dr. D. J. Dalgleish, Consultant Anaesthetist Royal Bournemouth and Christchurch Hospitals Trust, UK Questions 1. In the fetal circulation: a) There are two umbilical arteries and one umbilical vein? b) Over 90% of blood passes the liver via the ductus venosus c) The foramen ovale divides the left and right ventricle d) The umbilical artery carries oxygenated blood from the placenta to the fetus e) The foramen ovale allows oxygenated blood to bypass the pulmonary circulation 2. In the fetal circulation: a) The oxygen dissociation curve of fetal haemoglobin is shifted to the left compared with adult haemoglobin ensuring oxygen delivery to the fetus despite low oxygen partial pressures b) It is the presence of the ductus arteriosus and large pulmonary vascular resistance which ensures most of the right ventricular output passes into the aorta c) The patency of the ductus arteriosus is maintained by high oxygen tensions d) The patency of the ductus arteriosus is maintained by the vasodilating effects of prostaglandin G2 e) 2,3-DPG levels are higher in fetal haemoglobin compared with adult haemaglobin 3. Changes at birth include: a) a fall in pulmonary vascular resistance b) a rise in systemic vascular resistance with clamping of the cord c) an increase in hypoxic pulmonary vasoconstriction d) a rise in left atrial pressure e) closure of the ductus arteriosus within 24 hours 4. The following congenital heart lesions are cyanotic: a) Ventricular septal defect b) Atrial septal defect c) Patent ductus arteriosus d) Tetralogy of Fallot e) Transposition of the great arteries MCQ answers at end Key points • The fetal circulation supplies the fetal tissues with oxygen and nutrients from the placenta. -

The Pattern and Mechanisms of Response to Oxygen by the Ductus Arteriosus and Umbilical Artery
Pediat. Res. 6: 693-700 (1972) Acetylcholine neonate atropine prematurity bradykinin sympathetic nervous system ductus arteriosus The Pattern and Mechanisms of Response to Oxygen by the Ductus Arteriosus and Umbilical Artery INGRID OBERHANSLI-WEISS, MICHAEL A. HEYMANN1391, ABRAHAM M. RUDOLPH, AND KENNETH L. MELMON Cardiovascular Research Institute and Departments of Pediatrics, Physiology and Pharmacology, University of California San Francisco, San Francisco, California, USA Extract Response of the ductus arteriosus and umbilical artery to changes in oxygen tension, to acetylcholine, and to sympathetic and parasympathetic blocking agents was studied in vitro in isolated rings obtained from 22 fetal lambs of 98- to 147-day gestation. After stabilization of tension at a baseline level (0.3-0.7 g) in a PO2 environment of 35-45 mm Hg, both increase of the PO2 to 550 mm Hg and decrease of the PO2 to 8 mm Hg of the bathing solution produced constriction. The mean maximal tension developed by the ductus arteriosus.was 3.91 g at high PO2 and 3.87 g at low POr The increase in maximal tension developed with advancing gestation was also similar at both high and low POj. At P02 levels of 8-550 mm Hg, acetylcholine produced a further increase in tension, whereas bradykinin only produced an increase in tension at high PO2- Alpha and beta sympathetic blockade had no effect on the constrictor response to oxygen. Atropine relaxed the ductus arteriosus and umbilical artery at both high and low Po2 levels; the degree of relaxation was related to drug concentration. Acetylcholin- esterase also relaxed the ductus arteriosus constricted by oxygen. -

Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure
Acta Cardiol Sin 2016;32:731-737 Brief Report doi: 10.6515/ACS20160205A Echocardiographic Follow-Up of Patent Foramen Ovale and the Factors Affecting Spontaneous Closure Ali Yildirim,1 Alperen Aydin,2 Tevfik Demir,1 Fatma Aydin,2 Birsen Ucar1 and Zubeyir Kilic1 Background: The aim of the present study was to evaluate the echocardiographic follow-up of patent foramen ovale, which is considered a potential etiological factor in various diseases, and to determine the factors affecting spontaneous closure. Methods: Between January 2000 and June 2012, records of 918 patients with patent foramen ovale were retrospectively reviewed. Patency of less than 3 mm around the fossa ovalis is called patent foramen ovale. Patients with cyanotic congenital heart diseases, severe heart valve disorders and severe hemodynamic left to right shunts were excluded from the study. The patients were divided into three groups based on age; 1 day-1 monthingroup1,1month-12monthsingroup2,andmorethan12monthsingroup3. Results: Of the 918 patients, 564 (61.4%) had spontaneous closure, 328 (35.8%) had patent foramen ovale continued, 15 (1.6%) patients had patent foramen ovale enlarged to 3-5 mm, 6 patients were enlarged to 5-8 mm, and in one patient patent foramen ovale reached to more than 8 mm size. Defect was spontaneously closed in 65.9% of the patients in group 1, 66.7% of the patients in group 2, and 52.3% of the patients in group 3. There was a negative correlation between the age of diagnosis and spontaneous closure (p < 0.05). Gender, prematurity and coexisting malformations such as patent ductus arteriosus and atrial septal aneurysm did not have any effect on spontaneous closure of patent foramen ovale (p > 0.05).