Volume 22 / No. 5 / 1991
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Novel Triadius-Like N4 Specie of Iron Nitride Compounds Under High
www.nature.com/scientificreports OPEN Novel triadius-like N4 specie of iron nitride compounds under high pressure Received: 11 May 2018 Yuanzheng Chen1, Xinyong Cai1, Hongyan Wang1, Hongbo Wang2 & Hui Wang2 Accepted: 2 July 2018 Various nitrogen species in nitrides are fascinating since they often appear with these nitride as Published: xx xx xxxx superconductors, hard materials, and high-energy density. As a typical complex, though iron nitride has been intensively studied, nitrogen species in the iron–nitrogen (Fe-N) compounds only have been confned to single atom (N) or molecule nitrogen (N2). Using a structure search method based on the CALYPSO methodology, unexpectedly, we here revealed two new stable high pressure (HP) states at 1:2 and 1:4 compositions with striking nitrogen species. The results show that the proposed FeN2 stabilizes by a break up of molecule N2 into a novel planar N4 unit (P63/mcm, >228 GPa) while FeN4 stabilizes by a infnite 1D linear nitrogen chains N∞ (P-1, >50 GPa; Cmmm, >250 GPa). In the intriguing N4 specie of P63/mcm-FeN2, we fnd that it possesses three equal N = N covalent bonds and forms a perfect triadius-like confguration being never reported before. This uniqueness gives rise to a set of remarkable properties for the crystal phase: it is identifed to have a good mechanical property and a potential for phonon-mediated superconductivity with a Tc of 4–8 K. This discovery puts the Fe-N system into a new class of desirable materials combining advanced mechanical properties and superconductivity. Nitrogen (N) is the most abundant element in the earth’s atmosphere and is one of the least studied elements regarding the composition of the Earth1. -
A Raman and Infrared Spectroscopic Analysis of the Phosphate Mineral
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 126 (2014) 164–169 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa A Raman and infrared spectroscopic analysis of the phosphate mineral wardite NaAl3(PO4)2(OH)4Á2(H2O) from Brazil ⇑ Ray L. Frost a, , Ricardo Scholz b, Andrés López a, Cristiano Lana b, Yunfei Xi a a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35400-00, Brazil highlights graphical abstract A wardite mineral sample from Lavra Da Ilha, Minas Gerais, Brazil was analysed. Using SEM with EDX and vibrational spectroscopy. The calculated formula is (Na0.97Ca0.03)R1.00Al3 (PO4)2(OH)4Á2(H2O). Observation of multiple bands supports the concept of non- equivalent phosphate units in the structure. article info abstract Article history: A wardite mineral sample from Lavra Da Ilha, Minas Gerais, Brazil has been examined by vibrational spec- Received 17 November 2013 troscopy. The mineral is unusual in that it belongs to a unique symmetry class, namely the tetragonal-tra- Received in revised form 7 January 2014 pezohedral group. The structure of wardite contains layers of corner-linked –OH bridged MO6 octahedra Accepted 2 February 2014 stacked along the tetragonal C-axis in a four-layer sequence and linked by PO groups. Consequentially Available online 15 February 2014 4 not all phosphate units are identical. -

A Vibrational Spectroscopic Study of the Phosphate Mineral Vantasselite
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 147 (2015) 185–192 Contents lists available at ScienceDirect Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy journal homepage: www.elsevier.com/locate/saa A vibrational spectroscopic study of the phosphate mineral vantasselite Al4(PO4)3(OH)3Á9H2O ⇑ Ray L. Frost a, , Ricardo Scholz b, Fernanda Maria Belotti c, Andrés López a, Frederick L. Theiss a a School of Chemistry, Physics and Mechanical Engineering, Science and Engineering Faculty, Queensland University of Technology, GPO Box 2434, Brisbane, Queensland 4001, Australia b Geology Department, School of Mines, Federal University of Ouro Preto, Campus Morro do Cruzeiro, Ouro Preto, MG 35,400-00, Brazil c Federal University of Itajubá, Campus Itabira, Itabira, MG 35,903-087, Brazil highlights graphical abstract We have studied the phosphate mineral vantasselite Al2(PO4)(OH)3Á3H2O. Using a combination of SEM with EDX and Raman and infrared spectroscopy. Chemical analysis shows a mineral containing Al, Fe and P. A comparison is made with other aluminum containing phosphate minerals. Vibrational spectroscopy offers a mechanism for the study of the molecular structure of vantasselite. article info abstract Article history: We have studied the phosphate mineral vantasselite Al4(PO4)3(OH)3Á9H2O using a combination of SEM Received 28 October 2014 with EDX and Raman and infrared spectroscopy. Qualitative chemical analysis shows Al, Fe and P. Received in revised form 15 January 2015 À1 3À Raman bands at 1013 and 1027 cm are assigned to the PO4 m1 symmetric stretching mode. The obser- Accepted 23 March 2015 vation of two bands suggests the non-equivalence of the phosphate units in the vantasselite structure. -

Fluorowardite, Naal3(PO4)2(OH)2F2·2H2O, the Fluorine Analog of Wardite from the Silver Coin Mine, Valmy, Nevada
American Mineralogist, Volume 99, pages 804–810, 2014 Fluorowardite, NaAl3(PO4)2(OH)2F22 Anthony R. KAmpf1,*, pAul m. AdAms2, RobeRt m. housley3 And GeoRGe R. RossmAn3 1Mineral Sciences Department, Natural History Museum of Los Angeles County, 900 Exposition Boulevard, Los Angeles, California 90007, U.S.A. 2126 South Helberta Avenue, #2, Redondo Beach, California 90277, U.S.A. 3Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125, U.S.A. AbstRAct Fluorowardite (IMA2012-016), NaAl3(PO4)2(OH)2F2·2H2O, the F analog of wardite, is a new mineral from the Silver Coin mine, Valmy, Iron Point district, Humboldt County, Nevada, U.S.A., where it oc- curs as a low-temperature secondary mineral in complex phosphate assemblages rich in Al, Na, and F. Fluorowardite forms colorless to white or cream-colored, tetragonal-pyramidal crystals up to 0.1 mm in diameter. The streak is white. Crystals are transparent to translucent, with vitreous to pearly luster. The Mohs hardness is about 5, the tenacity is brittle, the fracture is irregular, and crystals exhibit one perfect cleavage on {001}. The calculated density is 2.760 g/cm3 analyses (average of 8) provided: Na2O 6.27, CaO 1.74, MgO 0.42, Al2O3 35.21, Fe2O3 0.72, P2O5 32.49, As2O52O 13.35 (structure), total 94.74 wt%. The presence of H2O and OH and the absence of CO3 3+ anions) is: (Na0.87Ca0.13Mg0.04)(Al2.96Fe0.04)(P1.96As0.03)O8.12(OH)2.35F1.53·2H2O. Fluorowardite 3 is tetragonal, P41212, acV , and Z lines in the X-ray powder diffraction pattern are [dobsI)(hkl)]: 4.766(100)(004,103); 3.099(75) (211,203); 3.008(62)(115,212); 2.834(28)(204,213); 2.597(56)(205); 1.7628(32)(400,401); 1.6592(29) R1FoF) 62O) octahedra, PO4 tetrahedra, and NaO6(H2O)26 octahedra link by corner-sharing to form a square array. -

Dicionarioct.Pdf
McGraw-Hill Dictionary of Earth Science Second Edition McGraw-Hill New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto Copyright © 2003 by The McGraw-Hill Companies, Inc. All rights reserved. Manufactured in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be repro- duced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. 0-07-141798-2 The material in this eBook also appears in the print version of this title: 0-07-141045-7 All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. Where such designations appear in this book, they have been printed with initial caps. McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. For more information, please contact George Hoare, Special Sales, at [email protected] or (212) 904-4069. TERMS OF USE This is a copyrighted work and The McGraw-Hill Companies, Inc. (“McGraw- Hill”) and its licensors reserve all rights in and to the work. Use of this work is subject to these terms. Except as permitted under the Copyright Act of 1976 and the right to store and retrieve one copy of the work, you may not decom- pile, disassemble, reverse engineer, reproduce, modify, create derivative works based upon, transmit, distribute, disseminate, sell, publish or sublicense the work or any part of it without McGraw-Hill’s prior consent. -

Geology of the Pegmatites and Associated Rocks of Maine
DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIRECTOR BULLETIN 445 GEOLOGY OF THE PEGMATITES AND ASSOCIATED ROCKS OF MAINE INCLUDING FELDSPAR, QUARTZ, MICA, AND GEM DEPOSITS BY EDSON S. BASTIN WASHINGTON GOVERNMENT PRINTING OFFICE 1911 CONTENTS. Introduction.............................................................. 9 Definition of pegmatite...................................................... 10 Geographic distribution.................................................... 10 Geology.................................................................. 10 Bordering rocks....................................................... 10 Pegmatites in foliated rocks........................................ 11 General statement............................................ 11 Sedimentary foliates........................................... 11 Igneous foliates.....".......................................... 12 Pegmatites in massive granites.................................... 13 'Age.................................................................. 15 General character..................................................... 15 Mineral and chemical composition................................. 15 Mineral constituents.......................................... 15 Relative proportions of minerals............................... 18 Quartzose phases. ..............................^............. 18 Fluidal cavities............................................... 19 Sodium and lithium phases................................... 20 Muscovite -

Roscherite-Group Minerals from Brazil
■ ■ Roscherite-Group Minerals yÜÉÅ UÜté|Ä Daniel Atencio* and José M.V. Coutinho Instituto de Geociências, Universidade de São Paulo, Rua do Lago, 562, 05508-080 – São Paulo, SP, Brazil. *e-mail: [email protected] Luiz A.D. Menezes Filho Rua Esmeralda, 534 – Prado, 30410-080 - Belo Horizonte, MG, Brazil. INTRODUCTION The three currently recognized members of the roscherite group are roscherite (Mn2+ analog), zanazziite (Mg analog), and greifensteinite (Fe2+ analog). These three species are monoclinic but triclinic variations have also been described (Fanfani et al. 1977, Leavens et al. 1990). Previously reported Brazilian occurrences of roscherite-group minerals include the Sapucaia mine, Lavra do Ênio, Alto Serra Branca, the Córrego Frio pegmatite, the Lavra da Ilha pegmatite, and the Pirineus mine. We report here the following three additional occurrences: the Pomarolli farm, Lavra do Telírio, and São Geraldo do Baixio. We also note the existence of a fourth member of the group, an as-yet undescribed monoclinic Fe3+-dominant species with higher refractive indices. The formulas are as follows, including a possible formula for the new species: Roscherite Ca2Mn5Be4(PO4)6(OH)4 • 6H2O Zanazziite Ca2Mg5Be4(PO4)6(OH)4 • 6H2O 2+ Greifensteinite Ca2Fe 5Be4(PO4)6(OH)4 • 6H2O 3+ 3+ Fe -dominant Ca2Fe 3.33Be4(PO4)6(OH)4 • 6H2O ■ 1 ■ Axis, Volume 1, Number 6 (2005) www.MineralogicalRecord.com ■ ■ THE OCCURRENCES Alto Serra Branca, Pedra Lavrada, Paraíba Unanalyzed “roscherite” was reported by Farias and Silva (1986) from the Alto Serra Branca granite pegmatite, 11 km southwest of Pedra Lavrada, Paraíba state, associated with several other phosphates including triphylite, lithiophilite, amblygonite, tavorite, zwieselite, rockbridgeite, huréaulite, phosphosiderite, variscite, cyrilovite and mitridatite. -
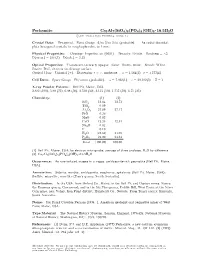
Perhamite Ca3al7(Sio4)3(PO4)4(OH)3 ² 16:5H2O C 2001 Mineral Data Publishing, Version 1.2 ° Crystal Data: Hexagonal
Perhamite Ca3Al7(SiO4)3(PO4)4(OH)3 ² 16:5H2O c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Hexagonal. Point Group: 6=m 2=m 2=m (probable). As radial discoidal, platy hexagonal crystals, in rough spherules, to 1 mm. Physical Properties: Cleavage: Imperfect on 0001 . Tenacity: Brittle. Hardness = 5 f g » D(meas.) = 2.64(1) D(calc.) = 2.53 Optical Properties: Translucent to nearly opaque. Color: Brown, white. Streak: White. Luster: Dull, vitreous on cleavage surface. Optical Class: Uniaxial (+). Dispersion: r > v; moderate. ! = 1.564(2) ² = 1.577(4) Cell Data: Space Group: P 6=mmm (probable). a = 7.022(1) c = 20.182(5) Z = 1 X-ray Powder Pattern: Bell Pit, Maine, USA. 2.882 (100), 5.80 (71), 6.08 (50), 3.510 (50), 3.115 (50), 1.757 (50), 6.71 (35) Chemistry: (1) (2) SiO2 13.64 13.72 TiO2 0.09 Al2O3 27.09 27.17 FeO 0.26 MgO 0.02 CaO 12.26 12.81 Na2O 0.02 F 0.10 H2O [24.62] 24.69 P2O5 21.90 21.61 Total [100.00] 100.00 (1) Bell Pit, Maine, USA; by electron microprobe, average of three analyses, H2O by di®erence. (2) Ca3Al7(SiO4)3(PO4)4(OH)3 ² 16:5H2O Occurrence: As rare isolated masses in a vuggy, amblygonite-rich pegmatite (Bell Pit, Maine, USA). Association: Siderite, wardite, amblygonite, eosphorite, sphalerite (Bell Pit, Maine, USA); °uellite, minyulite, wavellite (Tom's quarry, South Australia). Distribution: In the USA, from Oxford Co., Maine, in the Bell Pit and Dunton mines, Newry; the Emmons quarry, Greenwood; and in the Ski Pike quarry, Cobble Hill, West Paris; at the Silver Coin mine, near Valmy, Iron Point district, Humboldt Co., Nevada. -

Fluorowardite Naal3(PO4)2(OH)2F2·2H2O
Fluorowardite NaAl3(PO4)2(OH)2F2·2H2O Crystal Data: Tetragonal. Point Group: 422. As tetragonal bipyramidal crystals, to 0.1 mm, displaying {001} (prominent and lustrous), {011} and/or {012} (prominent, irregular, and striated parallel to [100]), and {100} (common, irregular, and striated parallel to [100]). Physical Properties: Cleavage: Perfect on {001}. Fracture: Irregular. Tenacity: Brittle. Hardness = 5 D(meas.) = n.d. D(calc.) = 2.706 Optical Properties: Transparent to translucent. Color: Colorless to white or cream-colored. Streak: White. Luster: Vitreous to pearly. Optical Class: Uniaxial (+). ω = 1.576(2) ε = 1.584(2) Cell Data: Space Group: P41212. a = 7.077(2) c = 19.227(3) Z = 4 X-ray Powder Pattern: Silver Coin mine, Valmy, Humboldt County, Nevada, USA. 4.766 (100), 3.099 (75), 3.008 (62), 2.597 (56), 1.5228 (49), 1.7628 (32), 1.6592 (29) Chemistry: (1) (2) Na2O 6.27 7.71 CaO 1.74 MgO 0.42 Al2O3 35.21 38.05 Fe2O3 0.72 P2O5 32.49 35.32 As2O5 0.64 F 6.76 9.45 -O = F2 2.85 3.98 H2O [13.35] 13.45 total 94.74 100.00 (1) Silver Coin mine, Valmy, Humboldt County, Nevada, USA; average of 8 electron microprobe analyses supplemented by FTIR and Raman spectroscopy, H2O calculated from the structure; 3+ corresponds to (Na0.87Ca0.13Mg0.04)Σ=1.04(Al2.96Fe 0.04)Σ=3.00(P1.96As0.03)Σ=1.99O8.12(OH)2.35F1.53·2H2O. (2) NaAl3(PO4)2(OH)2F2·2H2O. Occurrence: A secondary phase, in a F-rich secondary phosphate assemblage. -

Mineralogy of the Peraluminous Spruce Pine Plutonic Suite
MINERALOGY OF THE PERALUMINOUS SPRUCE PINE PLUTONIC SUITE, MITCHELL, AVERY, AND YANCEY COUNTIES, NORTH CAROLINA by William Brian Veal (Under the Direction of Samuel E. Swanson) ABSTRACT The Spruce Pine plutonic suite consists of numerous Devonian (390-410 ma) peraluminous granitic plutons, dikes, sills, and associated pegmatites. The mineralogy of pegmatites and host granodiorites from seven locations was studied to determine the extent of fractionation of the pegmatites relative to the granodiorite. Garnets in the plutons are almandine- spessartine and some display epitaxial overgrowths of grossular garnet. Similar garnets are found in the pegmatites. Pegmatitic muscovite contains several percent iron and is compositionally similar to muscovite from the host granodiorite. Feldspars in the granodiorites and pegmatites are compositionally similar (plagioclase Ab69-98; k-feldspar Or95-98). The lack of compositional variation between minerals in pegmatites and host granodiorites indicates the pegmatites were not the result of extreme fractionation and indicates that pegmatite mineralogy can be used to characterize the mineralogy of an entire granodiorite body. INDEX WORDS: Spruce Pine, Pegmatites, Granodiorite, Garnet, Muscovite, Feldspar MINERALOGY OF THE PERALUMINOUS SPRUCE PINE PLUTONIC SUITE, MITCHELL, AVERY, AND YANCEY COUNTIES, NORTH CAROLINA by WILLIAM BRIAN VEAL B.S., Georgia Southwestern State University, 2002 A Thesis Submitted to the Graduate Faculty of The University of Georgia in Partial Fulfillment of the Requirements for the Degree MASTER OF SCIENCE ATHENS, GEORGIA 2004 © 2004 William Brian Veal All Rights Reserved MINERALOGY OF THE PERALUMINOUS SPRUCE PINE PLUTONIC SUITE, MITCHELL, AVERY, AND YANCEY COUNTIES, NORTH CAROLINA by WILLIAM BRIAN VEAL Major Professor: Samual E. Swanson Committee: Michael F. -

Too Short-Lived Or Not Existing Species: N-Azidoamines
Article Cite This: J. Org. Chem. 2019, 84, 4033−4039 pubs.acs.org/joc Too Short-Lived or Not Existing Species: N‑Azidoamines Reinvestigated Klaus Banert* and Tom Pester Chemnitz University of Technology, Organic Chemistry, Strasse der Nationen 62, 09111 Chemnitz, Germany *S Supporting Information ABSTRACT: Treatment of N-chlorodimethylamine with sodium azide in dichloromethane does not lead to N-azidodimethylamine, as thought for more than 50 years. Instead, surprisingly, (azidomethyl)dimethylamine is generated with good reproducibility. A plausible reaction mechanism to explain the formation of this product is presented. The reaction of lithium dibenzylhy- drazide with tosyl azide does not result in the creation of an N-azidoamine, which can be detected by IR spectroscopy at ambient temperature, as it was claimed previously. Additional experiments with diazo group transfer to lithium hydrazides show that intermediate N-azidoamines are very short-lived or their formation is bypassed by direct generation of 1,1-diazenes via synchronous cleavage of two N−N bonds. 1. INTRODUCTION Scheme 1. Attempts To Generate N-Azidoamines The azido unit belongs to the most important functional groups in chemistry because of a variety of applications, particularly in the case of organic azides.1 Not only carbon but also most elements in the periodic table form a bond to azide;2 however, only a few of the corresponding covalent compounds are of general interest, for example, as reagents in synthetic chemistry, such as hydrazoic acid,3 silyl azides,4 phosphoryl azides,5 and sulfonyl azides.5b,6 In particular, azides attached to − another nitrogen atom have only rarely been reported.7a d Such azides, in which the azide-bearing additional nitrogen atom is always connected with at least one strongly electron- withdrawing group, turned out to be very unstable. -

Textures and Structures of Igneous Rocks
UNIT 2 TEXTURES AND STRUCTURES OF IGNEOUS ROCKS Structure______________________________________________ 2.1 Introduction 2.4 Forms of Igneous Rocks Expected Learning Outcomes Sill 2.2 Textures of Igneous Rocks Dyke Crystallinity Laccolith Granularity Bysmalith Shape of the Crystals Lopolith Mutual Relationship between Crystal and Phacolith Non-Crystalline Material Chonolith Intergrowth Textures Volcanic Neck Exsolution Textures Batholith Miscellaneous Textures Stock 2.3 Structures of Igneous Rocks Boss Vesicular and Amygdaloidal Structures 2.5 Summary Scoriaceous and Pumiceous Structures 2.6 Activity Lava Tunnels 2.7 Terminal Questions Blocky and Ropy Lava 2.8 References Platy and Sheet Structure 2.9 Further/Suggested Readings Pillow Lava 2.10 Answers Columnar/Prismatic Structure Lava Flow Structure Rift and Grain Perlitic Structure Rapakivi Structure Xenoliths ……………………………………………………………………………………………….…....Block 1 Igneous Petrology.......…-I 2.1 INTRODUCTION You have been introduced to the igneous rocks in the previous unit. You have also learnt that the slow cooling of magma takes place in the deeper parts of the Earth and large size crystals are formed. On the contrary, magma undergoing rapid cooling in shallower depth or on the surface of the Earth yielded fine grained crystals. The rapid cooling of lava/melt molten rock on the surface of the Earth produces fine grained minerals or glass. The igneous rocks vary in grain size from very coarse, medium to fine grained or even glassy in hand specimen and under the microscope. Thus, igneous rocks display variety of textures. Thus, the term texture refers to physical appearance of a rock. In this unit we will discuss textures and structures of igneous rocks.