Derivation of Immediately Dangerous to Life Or Health (IDLH) Values
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transport of Dangerous Goods
ST/SG/AC.10/1/Rev.16 (Vol.I) Recommendations on the TRANSPORT OF DANGEROUS GOODS Model Regulations Volume I Sixteenth revised edition UNITED NATIONS New York and Geneva, 2009 NOTE The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Secretariat of the United Nations concerning the legal status of any country, territory, city or area, or of its authorities, or concerning the delimitation of its frontiers or boundaries. ST/SG/AC.10/1/Rev.16 (Vol.I) Copyright © United Nations, 2009 All rights reserved. No part of this publication may, for sales purposes, be reproduced, stored in a retrieval system or transmitted in any form or by any means, electronic, electrostatic, magnetic tape, mechanical, photocopying or otherwise, without prior permission in writing from the United Nations. UNITED NATIONS Sales No. E.09.VIII.2 ISBN 978-92-1-139136-7 (complete set of two volumes) ISSN 1014-5753 Volumes I and II not to be sold separately FOREWORD The Recommendations on the Transport of Dangerous Goods are addressed to governments and to the international organizations concerned with safety in the transport of dangerous goods. The first version, prepared by the United Nations Economic and Social Council's Committee of Experts on the Transport of Dangerous Goods, was published in 1956 (ST/ECA/43-E/CN.2/170). In response to developments in technology and the changing needs of users, they have been regularly amended and updated at succeeding sessions of the Committee of Experts pursuant to Resolution 645 G (XXIII) of 26 April 1957 of the Economic and Social Council and subsequent resolutions. -
![Immediately Dangerous to Life Or Health (Idlh) Value Profile for Chlorine Pentafluoride [Cas No. 13637-63-3] and Bromine Pentafl](https://docslib.b-cdn.net/cover/6027/immediately-dangerous-to-life-or-health-idlh-value-profile-for-chlorine-pentafluoride-cas-no-13637-63-3-and-bromine-pentafl-116027.webp)
Immediately Dangerous to Life Or Health (Idlh) Value Profile for Chlorine Pentafluoride [Cas No. 13637-63-3] and Bromine Pentafl
External Review Draft March 2015 1 2 3 4 5 6 7 IMMEDIATELY DANGEROUS TO LIFE OR HEALTH (IDLH) VALUE PROFILE 8 9 10 11 FOR 12 13 14 15 CHLORINE PENTAFLUORIDE [CAS NO. 13637-63-3] 16 17 AND 18 19 BROMINE PENTAFLUORIDE [CAS NO. 7789-30-2] 20 21 22 23 24 25 26 Department of Health and Human Services 27 Centers for Disease Control and Prevention 28 National Institute for Occupational Safety and Health This information is distributed solely for the purpose of pre-dissemination peer review under applicable information quality guidelines. It has not been formally disseminated by the National Institute for Occupational Safety and Health. It does not represent and should not be construed to represent any agency determination or policy. i External Review Draft March 2015 1 DISCLAIMER 2 Mention of any company or product does not constitute endorsement by the National Institute for Occupational 3 Safety and Health (NIOSH). In addition, citations of Web sites external to NIOSH do not constitute NIOSH 4 endorsement of the sponsoring organizations or their programs or products. Furthermore, NIOSH is not 5 responsible for the content of these Web sites. 6 7 ORDERING INFORMATION 8 This document is in the public domain and may be freely copied or reprinted. To receive NIOSH documents or 9 other information about occupational safety and health topics, contact NIOSH at 10 Telephone: 1-800-CDC-INFO (1-800-232-4636) 11 TTY: 1-888-232-6348 12 E-mail: [email protected] 13 14 or visit the NIOSH Web site at www.cdc.gov/niosh. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Chemical Name Federal P Code CAS Registry Number Acutely
Acutely / Extremely Hazardous Waste List Federal P CAS Registry Acutely / Extremely Chemical Name Code Number Hazardous 4,7-Methano-1H-indene, 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-tetrahydro- P059 76-44-8 Acutely Hazardous 6,9-Methano-2,4,3-benzodioxathiepin, 6,7,8,9,10,10- hexachloro-1,5,5a,6,9,9a-hexahydro-, 3-oxide P050 115-29-7 Acutely Hazardous Methanimidamide, N,N-dimethyl-N'-[2-methyl-4-[[(methylamino)carbonyl]oxy]phenyl]- P197 17702-57-7 Acutely Hazardous 1-(o-Chlorophenyl)thiourea P026 5344-82-1 Acutely Hazardous 1-(o-Chlorophenyl)thiourea 5344-82-1 Extremely Hazardous 1,1,1-Trichloro-2, -bis(p-methoxyphenyl)ethane Extremely Hazardous 1,1a,2,2,3,3a,4,5,5,5a,5b,6-Dodecachlorooctahydro-1,3,4-metheno-1H-cyclobuta (cd) pentalene, Dechlorane Extremely Hazardous 1,1a,3,3a,4,5,5,5a,5b,6-Decachloro--octahydro-1,2,4-metheno-2H-cyclobuta (cd) pentalen-2- one, chlorecone Extremely Hazardous 1,1-Dimethylhydrazine 57-14-7 Extremely Hazardous 1,2,3,4,10,10-Hexachloro-6,7-epoxy-1,4,4,4a,5,6,7,8,8a-octahydro-1,4-endo-endo-5,8- dimethanonaph-thalene Extremely Hazardous 1,2,3-Propanetriol, trinitrate P081 55-63-0 Acutely Hazardous 1,2,3-Propanetriol, trinitrate 55-63-0 Extremely Hazardous 1,2,4,5,6,7,8,8-Octachloro-4,7-methano-3a,4,7,7a-tetra- hydro- indane Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]- 51-43-4 Extremely Hazardous 1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, P042 51-43-4 Acutely Hazardous 1,2-Dibromo-3-chloropropane 96-12-8 Extremely Hazardous 1,2-Propylenimine P067 75-55-8 Acutely Hazardous 1,2-Propylenimine 75-55-8 Extremely Hazardous 1,3,4,5,6,7,8,8-Octachloro-1,3,3a,4,7,7a-hexahydro-4,7-methanoisobenzofuran Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime 26419-73-8 Extremely Hazardous 1,3-Dithiolane-2-carboxaldehyde, 2,4-dimethyl-, O- [(methylamino)-carbonyl]oxime. -
Naming Molecular Compounds General Instructions: Please Do the Activities for Each Day As Indicated
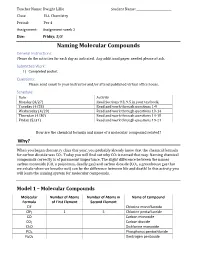
Teacher Name: Dwight Lillie Student Name: ________________________ Class: ELL Chemistry Period: Per 4 Assignment: Assignment week 2 Due: Friday, 5/8 Naming Molecular Compounds General Instructions: Please do the activities for each day as indicated. Any additional paper needed please attach. Submitted Work: 1) Completed packet. Questions: Please send email to your instructor and/or attend published virtual office hours. Schedule: Date Activity Monday (4/27) Read Sections 9.3, 9.5 in your textbook. Tuesday (4/28) Read and work through questions 1-9 Wednesday (4/29) Read and work through questions 10-14 Thursday (4/30) Read and work through questions 14-18 Friday (5/31) Read and work through questions 19-21 How are the chemical formula and name of a molecular compound related? Why? When you began chemistry class this year, you probably already knew that the chemical formula for carbon dioxide was CO2. Today you will find out why CO2 is named that way. Naming chemical compounds correctly is of paramount importance. The slight difference between the names carbon monoxide (CO, a poisonous, deadly gas) and carbon dioxide (CO2, a greenhouse gas that we exhale when we breathe out) can be the difference between life and death! In this activity you will learn the naming system for molecular compounds. Model 1 – Molecular Compounds Molecular Number of Atoms Number of Atoms in Name of Compound Formula of First Element Second Element ClF Chlorine monofluoride ClF5 1 5 Chlorine pentafluoride CO Carbon monoxide CO2 Carbon dioxide Cl2O Dichlorine monoxide PCl5 Phosphorus pentachloride N2O5 Dinitrogen pentoxide 1. Fill in the table to indicate the number of atoms of each type in the molecular formula. -

HIGH HAZARD GAS Review Date: 09/23/2019
University of Pittsburgh EH&S Guideline Number: 04-021 Safety Manual Subject: Effective Date: 04/19/2017 Page 1 of 9 HIGH HAZARD GAS Review Date: 09/23/2019 STORAGE AND USE OF HIGH HAZARD GAS 1. Definition of High Hazard (HH) Gases For these guidelines, any gas meeting one or more of the following definitions based on International Fire Code (IFC) and National Fire Protection Association (NFPA) standards: 1.1. Flammable gas – a material that is a gas at 68ºF (20ºC) or less at an absolute pressure of 14.7 psi (101.325 kPa) when in a mixture of 13% or less by volume with air, or that has a flammable range at an absolute pressure of 14.7 psi (101.325 kPa) with air of at least 12%, regardless of the lower limit 1.2. Pyrophoric gas – a gas with an autoignition temperature in air at or below 130ºF (54.4ºC) 1.3. Health Hazard 3 (HH3) gas – material that, under emergency conditions and according to the standards, can cause serious or permanent injury 1.4. Health Hazard 4 (HH4) gas – material that, under emergency conditions and according to the standards, can be lethal The storage and usage of a gas or gases meeting any of the above definitions must follow all applicable IFC and NFPA guidelines and the requirements outlined in this document. Consult EH&S for specific guidance on gas mixtures containing corrosive, flammable or poisonous gas components (ex. 1% carbon monoxide/nitrogen, 5% hydrogen sulfide/helium). 2. Notification Requirements Prior to Obtaining High Hazard Gases 2.1. -

IDLH) Value Profile
CAS® No. 13637-63-3 DEPARTMENT OF HEALTH AND HUMAN SERVICES Center for Disease Control and Prevention National Institute of Occupational Safety and Health This page intentionally left blank. Immediately Dangerous to Life or Health (IDLH) Value Profile Chlorine Pentafluoride [CAS® no. 13637-63-3] F F F Cl F F DEPARTMENT OF HEALTH AND HUMAN SERVICES Centers for Disease Control and Prevention National Institute for Occupational Safety and Health This document is in the public domain and may be freely copied or reprinted. Disclaimer Mention of any company or product does not constitute endorsement by the National Institute for Occupational Safety and Health (NIOSH). In addition, citations of websites external to NIOSH do not constitute NIOSH endorsement of the sponsoring organizations or their programs or prod- ucts. Furthermore, NIOSH is not responsible for the content of these websites. Ordering Information This document is in the public domain and may be freely copied or reprinted. To receive NIOSH documents or other information about occupational safety and health topics, contact NIOSH at Telephone: 1-800-CDC-INFO (1-800-232-4636) TTY: 1-888-232-6348 E-mail: [email protected] or visit the NIOSH website at www.cdc.gov/niosh. For a monthly update on news at NIOSH, subscribe to NIOSH eNews by visiting www.cdc.gov/niosh/eNews. Suggested Citation NIOSH [2016]. Immediately dangerous to life or health (IDLH) value profile: chlorine pentafluo- ride. By Dotson GS, Maier A, Parker A, Haber L. Cincinnati, OH: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupation- al Safety and Health, DHHS (NIOSH) Publication 2016-170. -

TIH/PIH List
Hazardous Materials Designated as TIH/PIH (consolidated AAR and Railinc lists) 3/12/2007 STCC Proper Shipping Name 4921402 2-CHLOROETHANAL 4921495 2-METHYL-2-HEPTANETHIOL 4921741 3,5-DICHLORO-2,4,6-TRIFLUOROPYRIDINE 4921401 ACETONE CYANOHYDRIN, STABILIZED 4927007 ACROLEIN, STABILIZED 4921019 ALLYL ALCOHOL 4923113 ALLYL CHLOROFORMATE 4921004 ALLYLAMINE 4904211 AMMONIA SOLUTION 4920360 AMMONIA SOLUTIONS 4904209 AMMONIA, ANHYDROUS 4904210 AMMONIA, ANHYDROUS 4904879 AMMONIA, ANHYDROUS 4920359 AMMONIA, ANHYDROUS 4923209 ARSENIC TRICHLORIDE 4920135 ARSINE 4932010 BORON TRIBROMIDE 4920349 BORON TRICHLORIDE 4920522 BORON TRIFLUORIDE 4936110 BROMINE 4920715 BROMINE CHLORIDE 4918505 BROMINE PENTAFLUORIDE 4936106 BROMINE SOLUTIONS 4918507 BROMINE TRIFLUORIDE 4921727 BROMOACETONE 4920343 CARBON MONOXIDE AND HYDROGEN MIXTURE, COMPRESSED 4920399 CARBON MONOXIDE, COMPRESSED 4920511 CARBON MONOXIDE, REFRIGERATED LIQUID 4920559 CARBONYL FLUORIDE 4920351 CARBONYL SULFIDE 4920523 CHLORINE 4920189 CHLORINE PENTAFLUORIDE 4920352 CHLORINE TRIFLUORIDE 4921558 CHLOROACETONE, STABILIZED 4921009 CHLOROACETONITRILE 4923117 CHLOROACETYL CHLORIDE 4921414 CHLOROPICRIN 4920516 CHLOROPICRIN AND METHYL BROMIDE MIXTURES 4920547 CHLOROPICRIN AND METHYL BROMIDE MIXTURES 4920392 CHLOROPICRIN AND METHYL CHLORIDE MIXTURES 4921746 CHLOROPIVALOYL CHLORIDE 4930204 CHLOROSULFONIC ACID 4920527 COAL GAS, COMPRESSED 4920102 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920303 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920304 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920305 COMMPRESSED GAS, TOXIC, FLAMMABLE, CORROSIVE, N.O.S. 4920101 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920300 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920301 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920324 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920331 COMPRESSED GAS, TOXIC, CORROSIVE, N.O.S. 4920165 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. 4920378 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. 4920379 COMPRESSED GAS, TOXIC, FLAMMABLE, N.O.S. -
![Appendix 3-3 Ignition / Explosion Risk Caused by Blending [Safety Guidelines for Chemical Experiments, 4Th Edition] by the Chemical Society of Japan, Maruzen (1999)](https://docslib.b-cdn.net/cover/1640/appendix-3-3-ignition-explosion-risk-caused-by-blending-safety-guidelines-for-chemical-experiments-4th-edition-by-the-chemical-society-of-japan-maruzen-1999-3001640.webp)
Appendix 3-3 Ignition / Explosion Risk Caused by Blending [Safety Guidelines for Chemical Experiments, 4Th Edition] by the Chemical Society of Japan, Maruzen (1999)
Supplement 1. Safe Handling of Hazardous Substances Appendix 3-3 Ignition / Explosion Risk Caused by Blending [Safety Guidelines for Chemical Experiments, 4th Edition] by the Chemical Society of Japan, Maruzen (1999) This table has been classified and structured according to the chemical structure based on the NFPA’s (National Fire Protection Association) “Manual of Hazardous Chemical Reaction 491M (1975),” in which examples of accident cases and hazardous reaction cases involving chemical substances blending have been compiled. 1. Oxidizing Substance & Flammable Substance 1) Oxidizing Substance 2) Flammable Substance a) oxo-halogen acid salt a) non-metal elemental substance perchlorate, chlorate, bromate, iodate, chlorite, phosphorus, sulfur, activated carbon, etc. hypochlorite, etc. b) metallic peroxide, hydrogen peroxide b) metal metallic peroxide: potassium peroxide, calcium magnesium, zinc, aluminum, etc. peroxide, etc. c) permanganate c) sulfide potassium permanganate, etc. phosphorus sulfide, antimony sulfide, carbon bisulfide, etc. d) nichrome acid salt d) hydride dichromate potassium, etc. silane, phosphine, dibrane, arsine, etc. e) nitrate salt e) carbide potassium nitrate, sodium nitrate, ammonium calcium carbide nitrate, etc. f) nitric acid, fuming nitric acid f) organic substance hydrocarbon, alcohol, ketone, organic acid, amine, etc. g) nitric acid, fuming nitric acid, chlorosulfuric g) other acid metal amid, cyanide, hydroxylamine, etc. h) chrome oxide (III) i) perchloric acid j) peroxodisulfuric acid k) chlorine oxide, chlorine dioxide, chlorine monoxide l) nitrogen dioxide (nitrogen tetroxide) m) halogen fluorine, chlorine, bromine, iodine, chlorine trifluoride, bromine trifluoride, iodine trifluoride, chlorine pentafluoride, bromine pentafluoride, iodine pentafluoride n) halogenation nitrogen nitrogen trifluoride, nitrogen trichloride, nitrogen tribromide, nitrogen triiodide 2. Hydrogen Peroxide & Metallic Oxide 3. Persulfate & Manganese metallic oxide managanese dioxide, mercuric oxide 4. -

REDOX and ADDITION REACTIONS of BINARY FLUORIDES a Thesis Submitted to the University of Glasgow in Fulfilment of the Requiremen
REDOX AND ADDITION REACTIONS OF BINARY FLUORIDES A thesis submitted to the University of Glasgow in fulfilment of the requirements for the degree of DOCTOR OF PHILOSOPHY by JOHN ALBERT BERRY B.Sc, Department of Chemistry, University of Glasgow, GLASGOW, December. 1976. ProQuest Number: 13804100 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a com plete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest ProQuest 13804100 Published by ProQuest LLC(2018). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States C ode Microform Edition © ProQuest LLC. ProQuest LLC. 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106- 1346 "There is something fascinating about science. One gets such wholesale returns of conjecture out of such a trifling investment of fact" Mark Twain "It is better to meet a mother bear robbed of her cubs than to meet some fool busy with a stupid project". Proverbs, 1£, 12 (Good News Bible) Acknowledgements I wish to express my sincere gratitude to my supervisors, Professor D.W.A, Sharp and Dr, J,M, Winfield for their help, encouragement and patience throughout this work, I should like to acknowledge the help and encourage ment I received from Dr, A, Prescott, especially with the work involving UF^ in CH^CN, I should also like to thank all my research student colleagues and the many members of staff who helped me, in particular Drs. -

The Chemical List of Interest
List of Toxic and Pyrophoric Gases that require preappro val from MSU EHS BEFORE purc hase Chemical MSDS CAS # Health Fire Reactive 1,3-BUTADIENE 1,3-BUTADIENE 106-99-0 2d 4 0 2-METHYL-1,3-BUTADIENE 2-METHYL-1,3-BUTADIENE 78-79-5 1i 4 0 ACETYL FLUORIDE ACETYL FLUORIDE 557-99-3 3 0 0 AMMONIA AMMONIA 7664-41-7 3 1 0 ANTIMONY PENTAFLUORIDE ANTIMONY PENTAFLUORIDE 7783-70-2 4 0 1 ARSENIC PENTAFLUORIDE ARSENIC PENTAFLUORIDE 7784-36-3 3 1 0 ARSENIC TRIFLUORIDE ARSENIC TRIFLUORIDE 7784-35-2 3 0 1 ARSINE ARSINE 7784-42-1 4 4 2 BIS(TRIFLUOROMETHYL)PEROXIDE BIS(TRIFLUOROMETHYL)PEROXIDE 927-84-4 a, j a, j a, j BORON TRIBROMIDE BORON TRIBROMIDE 10294-33-4 4 2 0 BORON TRICHLORIDE BORON TRICHLORIDE 10294-34-5 3 0 1 BORON TRIFLUORIDE BORON TRIFLUORIDE 7637-07-2 4 0 1 BROMINE BROMINE 7726-95-6 3 0 0 BROMINE CHLORIDE BROMINE CHLORIDE 13863-41-7 3 0 1 BROMINE PENTAFLUORIDE BROMINE PENTAFLUORIDE 7789-30-2 3 0 3 BROMINE TRIFLUORIDE BROMINE TRIFLUORIDE 7787-71-5 3 0 3 BROMOETHENE BROMOETHENE 593-60-2 2d 4 1 BROMOMETHANE BROMOMETHANE 74-83-9 3 1 0 CARBON DISULFIDE CARBON DISULFIDE 75-15-0 3 4 0 CARBON MONOXIDE CARBON MONOXIDE 630-08-0 2e 4 0 CARBONYL FLUORIDE CARBONYL FLUORIDE 353-50-4 4 0 1 CARBONYL SULFIDE CARBONYL SULFIDE 463-58-1 3 4 1 CHLORINE CHLORINE 7782-50-5 4 0 0 CHLORINE DIOXIDE CHLORINE DIOXIDE 10049-04-4 3 0 4 CHLORINE MONOXIDE CHLORINE MONOXIDE 12301-79-0 a a a CHLORINE PENTAFLUORIDE CHLORINE PENTAFLUORIDE 13637-63-3 3 0 3 CHLORINE TRIFLUORIDE CHLORINE TRIFLUORIDE 7790-91-2 4 0 3 CHLOROTRIFLUOROETHYLENE CHLOROTRIFLUOROETHYLENE 79-38-9 3 4 3 CARBON -

Chlorine Pentafluoride Hazard Summary
Common Name: CHLORINE PENTAFLUORIDE CAS Number: 13637-63-3 RTK Substance number: 0369 DOT Number: UN 2548 Date: July 2000 ----------------------------------------------------------------------- ----------------------------------------------------------------------- HAZARD SUMMARY WORKPLACE EXPOSURE LIMITS * Chlorine Pentafluoride can affect you when breathed in. The following exposure limits are for Hydrogen Fluoride * Chlorine Pentafluoride is a CORROSIVE CHEMICAL (measured as Fluorine): and contact may severely irritate and burn the skin and eyes with possible eye damage. OSHA: The legal airborne permissible exposure limit * Chlorine Pentafluoride is a HIGHLY REACTIVE (PEL) is 3 ppm averaged over an 8-hour CHEMICAL and a DANGEROUS EXPLOSION workshift. HAZARD. NIOSH: The recommended airborne exposure limit is IDENTIFICATION 3 ppm averaged over a 10-hour workshift and Chlorine Pentafluoride is a colorless gas with a sweet odor. 6 ppm, not to be exceeded during any 15 minute It is used as a fluorinating agent and in pulp bleaching. work period. REASON FOR CITATION ACGIH: The recommended airborne exposure limit is * Chlorine Pentafluoride is on the Hazardous Substance 3 ppm, which should not be exceeded at any List because it is regulated by OSHA and cited by time. ACGIH, DOT and NIOSH. * This chemical is on the Special Health Hazard Substance WAYS OF REDUCING EXPOSURE List because it is CORROSIVE and REACTIVE. * Where possible, enclose operations and use local exhaust * Definitions are provided on page 5. ventilation at the site of chemical release. If local exhaust ventilation or enclosure is not used, respirators should be HOW TO DETERMINE IF YOU ARE BEING worn. EXPOSED * Wear protective work clothing. The New Jersey Right to Know Act requires most employers * Wash thoroughly immediately after exposure to Chlorine to label chemicals in the workplace and requires public Pentafluoride and at the end of the workshift.