The Heteropolymolybdate Family: Structural Relations, Nomenclature Scheme and New Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Mineraiogy, Lithostratigraphy and Geochemistry of North Lngebright Lake, Saskatchewan, Canada
Mineraiogy, Lithostratigraphy and Geochemistry of North lngebright Lake, Saskatchewan, Canada BY Yuqiang Shang A Thesis Submitted to the Faculty of Graduate Studies in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Department of Geological Sciences University of Manitoba Winnipeg, Manitoba (c) December, 2000 National Library Bibliothèque nationale 1+1 ,,,da du Canada Acquisitions and Acquisitions et Bibliographic Services seMces bibliographiques 395 Weliington Street 395. rue Wellington OttawaON KlAON4 Oîtawa ON KIA ON4 Canada Canada The author has granted a non- L'auteur a accordé une licence non exclusive licence allowing the exclusive permettant a la National Library of Canada to Bhliotheque nationale du Canada de reproduce, loan, distri'bute or sell reproduire, prêter, distribuer ou copies of this thesis in microform, vendre des copies de cette thèse sous paper or electronic formats. la forme de microfiche/nlm, de reproduction sur papier ou sur format électronique. The author retains ownership of the L'auteur conserve la propriété du copyright in this thesis. Neither the droit d'auteur qui protège cette thèse. thesis nor substantial extracts from it Ni la thèse ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent être imp~imés reproduced without the author's ou autrement reproduits sans son permission. autorisation. THE UNIVERSITY OF MANITOBA FACULTY OF GRADUATE STUDIES ***** COPYRIGHT PERMISSION PAGE MineraIogy, Lithostratigraphy and Geochemistry of North Iigebright Lake, -

Leightonite K2ca2cu(SO4)4 • 2H2O C 2001-2005 Mineral Data Publishing, Version 1
Leightonite K2Ca2Cu(SO4)4 • 2H2O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Orthorhombic. Point Group: 2/m 2/m 2/m. Lathlike crystals, flattened on {100}, elongated along [010], or equant, showing {100}, {101}, {110}, {113}, several other forms, to 4 mm; commonly forms cross-fiber veinlets. Twinning: On {201}. Physical Properties: Hardness = 3 D(meas.) = 2.95 D(calc.) = 2.95 Optical Properties: Transparent to translucent. Color: Pale watery blue to greenish blue; pale blue in transmitted light. Luster: Vitreous. Optical Class: Biaxial (–). Orientation: X = c; Y = b; Z = a. Dispersion: r> v,moderately strong. α = 1.574–1.578 β = 1.587 γ = 1.595 2V(meas.) = ∼60◦ 2V(calc.) = 89◦060 Cell Data: Space Group: F mmm. a = 11.654(2) b = 7.497(1) c = 10.097(1) β = 125.21(1)◦ Z=2 X-ray Powder Pattern: Chuquicamata, Chile. 2.90 (100), 3.18 (60), 1.781 (30), 2.22 (20), 2.51 (10), 2.40 (10), 1.461 (10) Chemistry: (1) (2) SO3 49.33 49.87 CuO 11.97 12.39 CaO 18.41 17.46 Na2O 0.56 K2O 13.93 14.67 H2O 5.71 5.61 Total 99.91 100.00 • (1) Chuquicamata, Chile; corresponds to (K1.92Na0.12)Σ=2.04Ca2.13Cu0.98(SO4)4.00 2.06H2O. • (2) K2Ca2Cu(SO4)4 2H2O. Occurrence: Of localized occurrence in the oxidized zone of a copper deposit, probably formed under conditions of low acidity (Chuquicamata, Chile). Association: Natrochalcite, bl¨odite, atacamite, bellingerite, kr¨ohnkite,gypsum, quartz (Chuquicamata, Chile); chalcanthite, anhydrite, lammerite (Tsumeb, Namibia). -

Lammerite Cu3(Aso4,PO4)
Lammerite Cu3(AsO4, PO4)2 c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Monoclinic. Point Group: 2/m. Crystals, to 2 mm, are prismatic along [001] to tabular on {100} and terminated by {011}; additional forms include {010} and striated {120}; in radial spheroidal aggregates. Physical Properties: Cleavage: Perfect on {010}; good on {100}; imperfect on {001}. Hardness = 3.5–4 D(meas.) = 4.9–5.18 D(calc.) = 5.263 Optical Properties: Transparent. Color: Dark green. Streak: Pale green. Luster: Adamantine, vitreous on cleavage surfaces. Optical Class: Biaxial (+). Pleochroism: Strong; X = pale blue; Y = sky-blue; Z = pale bluish green. Orientation: X = b; Z ∧ c ' 40◦. Dispersion: rv, very strong. α = ∼1.89 β = 1.90–2.06 γ = 1.95–2.14 2V(meas.) = 54(5)◦ 2V(calc.) = 50◦ Cell Data: Space Group: P 21/a. a = 5.079(1) b = 11.611(2) c = 5.394(1) β = 111.72(2)◦ Z=2 X-ray Powder Pattern: Laurani, Bolivia. 2.89 (10), 2.52 (9), 3.06 (8), 3.00 (8), 2.62 (8), 2.59 (8), 2.84 (7) Chemistry: (1) (2) As2O5 49.8 32.64 P2O5 12.40 FeO 0.2 0.06 CuO 49.9 54.64 ZnO 0.8 0.11 MgO 0.2 Total 100.9 99.85 1− (1) Laurani, Bolivia; by electron microprobe, absence of (OH) and H2O confirmed by IR; corresponding to (Cu2.90Zn0.05Mg0.02Fe0.01)Σ=2.98(As1.00O4)2. (2) Tolbachik fissure volcano, Russia; by electron microprobe, corresponding to (Cu2.96Zn0.01)Σ=2.97[(As0.62P0.38)Σ=1.00O4]2. -

Kunatite, Cufe (PO) (OR) °4R 0, a New Member of The
Kunatite, CuFe (PO) (OR) °4R 0, a new 2 4 2 2 2 member ofthe whitmoreite group, from Lake Boga, Victoria, Australia. Stuart J. Mills Department of Earth and Ocean Sciences, University of British Columbia, Vancouver Be, Canada, V6T 1Z4 email: [email protected] Uwe Kolitsch Mineralogisch-Petrographische Abteilung, Naturhistorisches Museum, Burgring 7, A-10lO, Wien, Austria William D. Birch Department of Geosciences, Museum Victoria, GPO Box 666, Melbourne, Victoria 3001, Australia Jifi Sejkora Department of Mineralogy and Petrology, National Museum, Vaclavske nam. 68, CZ-1l5 79 Praha 1, Czech Republic ABSTRACT Kunatite, CuFe2(P04)2(OH)2'4HP, (IMA 2007--(57), is a newly defined mineral from a quarry in Late Devonian granite near Lake Boga, northern Victoria, Australia. The mineral occurs as acicular to lath-like microcrystals forming compact to slightly open spheres, hemispheres and flattened sprays up to about 0.25 mm across, with individual fibrous crystals up to 30 flm long and 5 flm thick. Kunatite is associated only with chalcosiderite-turquoise. The crystals are yellow-green with a pale yellow streak and are transparent; the lustre of the aggregates is silky and the estimated Mohs hardness is 3. Kunatite is monoclinic, P2/c, with a = 9.863(10), b = 9.661(6), c = 5.476(6) A, ~ = 92.45(3)°, V = 521.3(3) A3 and Z = 2. The calculated density, based on the empirical formula, is 3.063 3 • = ~ = = = g/cm Crystals are biaxial (-), with a 1.703(3), 1.742(4) and y 1.762(3), and 2Vca1c 70°. Orientation: Z-c, with straight extinction and slight pleochroism: X = very pale yellow (almost colourless), Y = pale yellowish, Z = yellowish. -

A Specific Gravity Index for Minerats
A SPECIFICGRAVITY INDEX FOR MINERATS c. A. MURSKyI ern R. M. THOMPSON, Un'fuersityof Bri.ti,sh Col,umb,in,Voncouver, Canad,a This work was undertaken in order to provide a practical, and as far as possible,a complete list of specific gravities of minerals. An accurate speciflc cravity determination can usually be made quickly and this information when combined with other physical properties commonly leads to rapid mineral identification. Early complete but now outdated specific gravity lists are those of Miers given in his mineralogy textbook (1902),and Spencer(M,i,n. Mag.,2!, pp. 382-865,I}ZZ). A more recent list by Hurlbut (Dana's Manuatr of M,i,neral,ogy,LgE2) is incomplete and others are limited to rock forming minerals,Trdger (Tabel,l,enntr-optischen Best'i,mmungd,er geste,i,nsb.ildend,en M,ineral,e, 1952) and Morey (Encycto- ped,iaof Cherni,cal,Technol,ogy, Vol. 12, 19b4). In his mineral identification tables, smith (rd,entifi,cati,onand. qual,itatioe cherai,cal,anal,ys'i,s of mineral,s,second edition, New york, 19bB) groups minerals on the basis of specificgravity but in each of the twelve groups the minerals are listed in order of decreasinghardness. The present work should not be regarded as an index of all known minerals as the specificgravities of many minerals are unknown or known only approximately and are omitted from the current list. The list, in order of increasing specific gravity, includes all minerals without regard to other physical properties or to chemical composition. The designation I or II after the name indicates that the mineral falls in the classesof minerals describedin Dana Systemof M'ineralogyEdition 7, volume I (Native elements, sulphides, oxides, etc.) or II (Halides, carbonates, etc.) (L944 and 1951). -

Vanadium-Bearing Interlava Sediment from The
VANADIUM-BEARING INTERLAVA SEDIMENT FROM THE CAMPBELL RIVER AREA, BRITISH COLUMBIA BY JOHN LESLIE JAMBOR B.A. University of British Columbia, 1957 THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE In the Department of Geology We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA April, 1960 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the Head of my Department or by his representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission-. Department of The University of British Columbia, Vancouver 3, Canada. Date CfMJ X?. ABSTRACT Vanadium is concentrated in laminated, black carbonaceous, siliceous sedimentary rocks at Menzies Bay and Quadra Island, Campbell River area, British Columbia. The vanadiferous rocks are intercalated with amygdaloidal, porphyritic basalts, andesites, and spilites, many of which are pillowform. The writer has correlated the Menzies Bay, Vancouver Island, flows with the Upper Triassic Texada formation volcanic rocks of Quadra Island. A limited petrographic study of the Texada flows in the area has indicated that pumpellyite is copious and widely distributed. Amygdaloidal greenockite is present in trace amounts. The identification of pumpellyite,re• garded as amphibole by earlier writers, marks its first occurrence in British Columbia. In a detailed study of the mineralization assoc• iated with the vanadiferous sedimentary rocks, the first British Columbian occurrences were noted for tenorite, brochantite, and cyanotrichite. -

IMA–CNMNC Approved Mineral Symbols
Mineralogical Magazine (2021), 85, 291–320 doi:10.1180/mgm.2021.43 Article IMA–CNMNC approved mineral symbols Laurence N. Warr* Institute of Geography and Geology, University of Greifswald, 17487 Greifswald, Germany Abstract Several text symbol lists for common rock-forming minerals have been published over the last 40 years, but no internationally agreed standard has yet been established. This contribution presents the first International Mineralogical Association (IMA) Commission on New Minerals, Nomenclature and Classification (CNMNC) approved collection of 5744 mineral name abbreviations by combining four methods of nomenclature based on the Kretz symbol approach. The collection incorporates 991 previously defined abbreviations for mineral groups and species and presents a further 4753 new symbols that cover all currently listed IMA minerals. Adopting IMA– CNMNC approved symbols is considered a necessary step in standardising abbreviations by employing a system compatible with that used for symbolising the chemical elements. Keywords: nomenclature, mineral names, symbols, abbreviations, groups, species, elements, IMA, CNMNC (Received 28 November 2020; accepted 14 May 2021; Accepted Manuscript published online: 18 May 2021; Associate Editor: Anthony R Kampf) Introduction used collection proposed by Whitney and Evans (2010). Despite the availability of recommended abbreviations for the commonly Using text symbols for abbreviating the scientific names of the studied mineral species, to date < 18% of mineral names recog- chemical elements -

Notes, Abstracts and Reviews Are in Ordinary Type. Only Minerals for Which Definite Data Are Given Are Indexed
TNDEX TO VOLUME 23 Leading articles are in bold face type; notes, abstracts and reviews are in ordinary type. only minerals for which definite data are given are indexed. Acmite occurrence in riebeckite- Apatite-phlogopite deposits of microgranite.(Nockold)..... l2l Quebec. (Landes). 359 Actinolite. 371 Arshinov, V. V. Pocket mineralogi- Actinolitic amphibole from New cal or polarizing magnifier.. 287 Zealand.(Hutton).... .. 539 Artz,L. C. Occurrenceof wavellite, AdelieLand rocks.(Stewart)... .. 464 GilesCo.,Va..... 664 Afirondack garnet deposits, gene- Ascharite(F). (Godlevsky)....... 294 sisof. (Miller).. ... .....174,399 Asovskite. (Efremov). 667 Ahlfeld, F. and Reyes, J. M. Aspland,V.t.... ........ 415 Mineralogie von Bolivien. Atacamite. ........706 [Book review]. 538 Atomic packing models of some Almandite. ..351.816 common sillicate structures, Aluminum and silicosis. @mmons, @orris, tr'rondel, Gtissow, Fries). 654 Lopez, Lotd, Parrish, Shimer) Alunite. jS4 ......65,182 Amalgams, natural. @ennan, Har- Austinite and brickerite, identity court).. 761 of. (Brendler) 347 Amarantite. 747 Australites, a unique shower of Aminoffite. (Hurlbut) 293 glassmeteorites. (Fenner).. 4Ls Amphibolization of sills and dikes Autunite. 275 in Libby quadrangle, Mon- Axinite,notes on. (Peacock)..... SZ2 tana.(Gibson, Jenks). ..... 3Oz Azurite. 7lO Anauxites and kaolinites, struc- tural relationshipsof .(Machat- Bandy, M. C. Mineralogy of tlree schki). tl7 sulphate deposits of Northern Andalusite in pegrnatite from Chile... 669 Fresno, Co., Calif. (Macdon- with Peacock, M. A. Un- ald, Merriam) 58S gemachite and clino-unge- Anderson,B. W... Zg3 nachite, new minerals from Anderson, C. A. Volcanoes of Chile... 314 Medicine Lake Highland, Bandylite. 704 Calif.. 166 Bandylite and a:rtofagastite, new Andradite. 432 copper minerals from Chile. Anhydrite ....... 898 (Palache, Foshag). -

Thn AMERICAN M INERALOGIST of AMERICA JOURNAL of the MINERALOGICAL SOCIETY
THn AMERICAN M INERALOGIST OF AMERICA JOURNAL OF THE MINERALOGICAL SOCIETY No. 11 Vol. 23 NOVEMBER, 1938 MINERALOGY OF THREE SULPHATE DEPOSITS OF NORTHERN CHILE* Menr C. BaNov, HaraardUniaersity, Combri'd'ge, Moss' CoNrnNrs page Univer- * Based on a thesis submitted to the Division of Geological Sciences, Harvard of Philos- sity, in 1938, in partial fulfillment of the requirements for the degree of Doctor oPhY' 669 670 , MARK C. BANDV AssrRecr This paper gives the results of a study of the mineralogy of Chuquicamata, euerena, and Alcaparrosa, three sulphate deposits near calama, in Northern chile. The sulphate and chloride minerals, their description, their paragenesis, and the geochemistry of their for- mation, are the primary interest of the paper. The minerals described were collected by the author in 1935. Seventy-six minerals were identified and studied. Eighteen of these minerals are known only from northern chile and twelve are known only from these three deposits. An at- tempt has been made to clear up some of the varietal na:nes and doubtful species that have been described from these mines. Seven new well-defined mineral species were discovered during the research-antofagastite, bandylite, leightonite, ungemachite, and lindgrenite have been described elsewhere; metasideronatrite and parabutlerite are described here. For all of these. complete crystallographic, optical, and chemical data were secured. rm- portant new crystallographic data are presented for the minerals szomolnokite, pickering- ite, rhomboclase, botryogen, lapparentite and natrojarosite. New *+ay data were ob- tained on three minerals and new optical data on sixteen, and nine new chemical analyses are presented. PART I. -
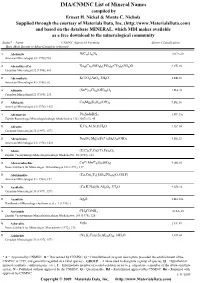
IMA/CNMNC List of Mineral Names
IMA/CNMNC List of Mineral Name s compiled by Ernest H. Nickel & Monte C. Nichols Supplied through the courtesy of Materials Data, Inc. (http://www.MaterialsData.com) and based on the database MINERAL, which MDI makes available as a free download to the mineralogical community Status* Name CNMNC Approved Formula Strunz Classification Best, Most Recent or Most Complete reference. A Abelsonite NiC£¡H£¢N¤ 10.CA.20 American Mineralogist 63 (1978) 930 A Abenakiite-(Ce) Na¢¦Ce¦(SiO£)¦(PO¤)¦(CO£)¦(SO¢)O 9.CK.10 Canadian Mineralogist 32 (1994), 843 G Abernathyite K(UO¢)AsO¤•3H¢O 8.EB.15 American Mineralogist 41 (1956), 82 A Abhurite (SnÀÈ)¢¡Cl¡¦(OH)¡¤O¦ 3.DA.30 Canadian Mineralogist 23 (1985), 233 D Abkhazite Ca¢Mg¥Si¨O¢¢(OH)¢ 9.DE.10 American Mineralogist 63 (1978), 1023 A Abramovite Pb¢SnInBiS§ 2.HF.25a Zapiski Rossiiskogo Mineralogicheskogo Obshchetstva 136 (2007) (5), 45 D Abrazite K,Ca,Al,Si,O,H¢O 9.GC.05 Canadian Mineralogist 35 (1997), 1571 D Abriachanite Na¢(Fe,Mg)£(FeÁÈ)¢Si¨O¢¢(OH)¢ 9.DE.25 American Mineralogist 63 (1978), 1023 D Absite (U,Ca,Y,Ce)(Ti,Fe)¢O¦ Zapiski Vsesoyuznogo Mineralogicheskogo Obshchestva 92 (1963), 113 A Abswurmbachite CuÀÈ(MnÁÈ)¦O¨(SiO¤) 9.AG.05 Neues Jahrbuch für Mineralogie, Abhandlungen 163 (1991), 117 D Abukumalite (Ca,Ce)¢Y£(SiO¤,PO¤)£(O,OH,F) American Mineralogist 51 (1966), 152 D Acadialite (Ca,K,Na)(Si,Al)£O¦•3H¢O 9.GD.10 Canadian Mineralogist 35 (1997), 1571 G Acanthite Ag¢S 2.BA.30a Handbook of Mineralogy (Anthony et al.), 1 (1990), 1 A Acetamide CH£CONH¢ 10.AA.20 Zapiski Vsesoyuznogo Mineralogicheskogo -

Wikipedia Writing Assignment Dr
Wikipedia Writing Assignment Dr. Elizabeth J. Catlos, Assoc. Professor, Jackson School of Geosciences, UT Austin This is a series of writing assignments I provided to my students enrolled in an Earth Materials course taught in the Dept. of Geological Sciences at UT Austin. Students complete a Wikipedia entry regarding a mineral that is currently missing from the on- line dictionary. The entry is developed throughout the semester as different topics regarding mineral properties are discussed in lecture. Overall, the course is geared towards introducing students to minerals, mineral study techniques, igneous and metamorphic rocks, ore deposits, and ore formation processes. Students take the class after passing introductory geology and basic chemistry. I decided to try the Wikipedia assignment after frustrations with an end-of-semester term paper, which I suspect most students started the night before it was due. My preferred assignment for Mineralogy courses I taught previously was to make a poster about an unusual mineral. Unfortunately this was not possible due to the large class size (>80 students). The assignment was 10% of their total grade (two in-class lecture exams =30%, lecture final=20%, lab exercises =10%; two lab exams= 30%). The first assignment: Students create an entry indicating a mineral’s chemical formula and origin of its name. They become familiar with Wikipedia and library resources available in Geology. The second assignment: Students create a table in Wikipedia preferred format for mineral entries that lists important characteristics. The third assignment: Students describe their mineral’s crystal class. They describe the meaning of the crystal class in their own words.