Pre-Motor Versus Motor Cerebral Cortex Neuromodulation for Chronic
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D
Cerebral Cortex Structure, Function, Dysfunction Reading Ch 10 Waxman Dental Neuroanatomy Lecture Suzanne Stensaas, Ph.D. March 15, 2011 Anatomy Review • Lobes and layers • Brodmann’s areas • Vascular Supply • Major Neurological Findings – Frontal, Parietal, Temporal, Occipital, Limbic • Quiz Questions? Types of Cortex • Sensory • Motor • Unimodal association • Multimodal association necessary for language, reason, plan, imagine, create Structure of neocortex (6 layers) The general pattern of primary, association and mulimodal association cortex (Mesulam) Brodmann, Lateral Left Hemisphere MCA left hemisphere from D.Haines ACA and PCA -Haines Issues of Functional Localization • Earliest studies -Signs, symptoms and note location • Electrical discharge (epilepsy) suggested function • Ablation - deficit suggest function • Reappearance of infant functions suggest loss of inhibition (disinhibition), i.e. grasp, suck, Babinski • Variabilities in case reports • Linked networks of afferent and efferent neurons in several regions working to accomplish a task • Functional imaging does not always equate with abnormal function associated with location of lesion • fMRI activation of several cortical regions • Same sign from lesions in different areas – i.e.paraphasias • Notion of the right hemisphere as "emotional" in contrast to the left one as "logical" has no basis in fact. Limbic System (not a true lobe) involves with cingulate gyrus and the • Hippocampus- short term memory • Amygdala- fear, agression, mating • Fornix pathway to hypothalamus • -

Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia
brain sciences Review Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper Motor Neuron Dominant Amyotrophic Lateral Sclerosis, and Hereditary Spastic Paraplegia Timothy Fullam and Jeffrey Statland * Department of Neurology, University of Kansas Medical Center, Kansas, KS 66160, USA; [email protected] * Correspondence: [email protected] Abstract: Following the exclusion of potentially reversible causes, the differential for those patients presenting with a predominant upper motor neuron syndrome includes primary lateral sclerosis (PLS), hereditary spastic paraplegia (HSP), or upper motor neuron dominant ALS (UMNdALS). Differentiation of these disorders in the early phases of disease remains challenging. While no single clinical or diagnostic tests is specific, there are several developing biomarkers and neuroimaging technologies which may help distinguish PLS from HSP and UMNdALS. Recent consensus diagnostic criteria and use of evolving technologies will allow more precise delineation of PLS from other upper motor neuron disorders and aid in the targeting of potentially disease-modifying therapeutics. Keywords: primary lateral sclerosis; amyotrophic lateral sclerosis; hereditary spastic paraplegia Citation: Fullam, T.; Statland, J. Upper Motor Neuron Disorders: Primary Lateral Sclerosis, Upper 1. Introduction Motor Neuron Dominant Jean-Martin Charcot (1825–1893) and Wilhelm Erb (1840–1921) are credited with first Amyotrophic Lateral Sclerosis, and describing a distinct clinical syndrome of upper motor neuron (UMN) tract degeneration in Hereditary Spastic Paraplegia. Brain isolation with symptoms including spasticity, hyperreflexia, and mild weakness [1,2]. Many Sci. 2021, 11, 611. https:// of the earliest described cases included cases of hereditary spastic paraplegia, amyotrophic doi.org/10.3390/brainsci11050611 lateral sclerosis, and underrecognized structural, infectious, or inflammatory etiologies for upper motor neuron dysfunction which have since become routinely diagnosed with the Academic Editors: P. -

Why Do Bridging Veins Rupture Into the Virtual Subdural Space?
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.47.2.121 on 1 February 1984. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1984;47:121-127 Why do bridging veins rupture into the virtual subdural space? T YAMASHIMA, RL FRIEDE From the Department ofNeuropathology, University of Gottingen, Gottingen, Federal Republic of Germany SUMMARY Electron microscopic data on human bridging veins show thin walls of variable thick- ness, circumferential arrangement of collagen fibres and a lack of outer reinforcement by arach- noid trabecules, all contributory to the subdural portion of the vein being more fragile than its subarachnoid portion. These features explain the laceration of veins and the subdural location of resultant haematomas. Most subdural haematomas due to venous bleeding walls are delicate, lacking muscle fibres, with only a have been attributed to lacerations in bridging veins. thin fibrous wall and a thin elastic lamina adjacent to These veins form short trunks passing directly from the endothelial layer. The conclusions of these two the brain to the dura mater, almost at right angles to authors, have gained wide acceptance, although guest. Protected by copyright. both. Between these two points, bridging veins take there was little evidence concerning the fragility of a straight course with no tortuosity to allow for the the vein walls. possible displacement of brain.' Trotter2 speculated The purpose of the present communication is to that subdural haematomas are invariably due to provide electron microscopic data on tissue fixed in trauma tearing large veins, an interpretation situ, which might throw some light on to the lacera- elaborated by Krauland.3 According to Leary,4 the tion mechanism of bridging veins and its relationship common sources of subdural haematomas are rup- to the development of subdural haematoma. -

Structure and Function of Visual Area MT
AR245-NE28-07 ARI 16 March 2005 1:3 V I E E W R S First published online as a Review in Advance on March 17, 2005 I E N C N A D V A Structure and Function of Visual Area MT Richard T. Born1 and David C. Bradley2 1Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115-5701; email: [email protected] 2Department of Psychology, University of Chicago, Chicago, Illinois 60637; email: [email protected] Annu. Rev. Neurosci. Key Words 2005. 28:157–89 extrastriate, motion perception, center-surround antagonism, doi: 10.1146/ magnocellular, structure-from-motion, aperture problem by HARVARD COLLEGE on 04/14/05. For personal use only. annurev.neuro.26.041002.131052 Copyright c 2005 by Abstract Annual Reviews. All rights reserved The small visual area known as MT or V5 has played a major role in 0147-006X/05/0721- our understanding of the primate cerebral cortex. This area has been 0157$20.00 historically important in the concept of cortical processing streams and the idea that different visual areas constitute highly specialized Annu. Rev. Neurosci. 0.0:${article.fPage}-${article.lPage}. Downloaded from arjournals.annualreviews.org representations of visual information. MT has also proven to be a fer- tile culture dish—full of direction- and disparity-selective neurons— exploited by many labs to study the neural circuits underlying com- putations of motion and depth and to examine the relationship be- tween neural activity and perception. Here we attempt a synthetic overview of the rich literature on MT with the goal of answering the question, What does MT do? www.annualreviews.org · Structure and Function of Area MT 157 AR245-NE28-07 ARI 16 March 2005 1:3 pathway. -

Prefrontal and Posterior Parietal Contributions to the Perceptual Awareness of Touch M
www.nature.com/scientificreports OPEN Prefrontal and posterior parietal contributions to the perceptual awareness of touch M. Rullmann1,2,5, S. Preusser1,5 & B. Pleger1,3,4* Which brain regions contribute to the perceptual awareness of touch remains largely unclear. We collected structural magnetic resonance imaging scans and neurological examination reports of 70 patients with brain injuries or stroke in S1 extending into adjacent parietal, temporal or pre-/frontal regions. We applied voxel-based lesion-symptom mapping to identify brain areas that overlap with an impaired touch perception (i.e., hypoesthesia). As expected, patients with hypoesthesia (n = 43) presented lesions in all Brodmann areas in S1 on postcentral gyrus (BA 1, 2, 3a, 3b). At the anterior border to BA 3b, we additionally identifed motor area BA 4p in association with hypoesthesia, as well as further ventrally the ventral premotor cortex (BA 6, BA 44), assumed to be involved in whole-body perception. At the posterior border to S1, we found hypoesthesia associated efects in attention-related areas such as the inferior parietal lobe and intraparietal sulcus. Downstream to S1, we replicated previously reported lesion-hypoesthesia associations in the parietal operculum and insular cortex (i.e., ventral pathway of somatosensory processing). The present fndings extend this pathway from S1 to the insular cortex by prefrontal and posterior parietal areas involved in multisensory integration and attention processes. Te primary somatosensory cortex (S1) in monkeys can be divided into four Brodmann areas: (BA) 1, 2, 3a, and 3b. Each BA consists of a somatotopically organized map that subserves distinct somatosensory functions1–3. -

3D Histological Reconstruction of Fiber Tracts and Direct Comparison with Diffusion Tensor MRI Tractography
3D Histological Reconstruction of Fiber Tracts and Direct Comparison with Diffusion Tensor MRI Tractography Julien Dauguet1, Sharon Peled2, Vladimir Berezovskii3, Thierry Delzescaux4, Simon K. Warfield1, Richard Born3, and Carl-Fredrik Westin5 1 Computational Radiology Laboratory, Children’s Hospital, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA 2 Harvard Center for Neurodegeneration and Repair, Boston, USA 3 Department of Neurobiology, Harvard Medical School, Boston, USA 4 Service Hospitalier Fr´ed´eric Joliot, CEA, Orsay, France 5 Laboratory of Mathematics in Imaging, Brigham and Women’s Hospital, Harvard Medical School, Boston, USA Abstract. A classical neural tract tracer, WGA-HRP, was injected at multiple sites within the brain of a macaque monkey. Histological sections of the labeled fiber tracts were reconstructed in 3D, and the fibers were segmented and registered with the anatomical post-mortem MRI from the same animal. Fiber tracing along the same pathways was performed on the DTI data using a classical diffusion tracing technique. The fibers derived from the DTI were compared with those segmented from the histology in order to evaluate the performance of DTI fiber tracing. While there was generally good agreement between the two methods, our results reveal certain limitations of DTI tractography, particularly at regions of fiber tract crossing or bifurcation. 1 Introduction Tracing neural connections lies at the heart of neuroanatomy, and has profound implications for the study of neural function and for the study of developmental and adult plasticity of the nervous system. It is also important for the study of neurodegenerative diseases, and for the planning of neurosurgical procedures, such as tumor ablation. -

Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans Ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai
Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai To cite this version: Hans ten Donkelaar, Nathalie Tzourio-Mazoyer, Jürgen Mai. Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex. Frontiers in Neuroanatomy, Frontiers, 2018, 12, pp.93. 10.3389/fnana.2018.00093. hal-01929541 HAL Id: hal-01929541 https://hal.archives-ouvertes.fr/hal-01929541 Submitted on 21 Nov 2018 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. REVIEW published: 19 November 2018 doi: 10.3389/fnana.2018.00093 Toward a Common Terminology for the Gyri and Sulci of the Human Cerebral Cortex Hans J. ten Donkelaar 1*†, Nathalie Tzourio-Mazoyer 2† and Jürgen K. Mai 3† 1 Department of Neurology, Donders Center for Medical Neuroscience, Radboud University Medical Center, Nijmegen, Netherlands, 2 IMN Institut des Maladies Neurodégénératives UMR 5293, Université de Bordeaux, Bordeaux, France, 3 Institute for Anatomy, Heinrich Heine University, Düsseldorf, Germany The gyri and sulci of the human brain were defined by pioneers such as Louis-Pierre Gratiolet and Alexander Ecker, and extensified by, among others, Dejerine (1895) and von Economo and Koskinas (1925). -

01 05 Lateral Surface of the Brain-NOTES.Pdf
Lateral Surface of the Brain Medical Neuroscience | Tutorial Notes Lateral Surface of the Brain 1 MAP TO NEUROSCIENCE CORE CONCEPTS NCC1. The brain is the body's most complex organ. LEARNING OBJECTIVES After study of the assigned learning materials, the student will: 1. Demonstrate the four paired lobes of the cerebral cortex and describe the boundaries of each. 2. Sketch the major features of each cerebral lobe, as seen from the lateral view, identifying major gyri and sulci that characterize each lobe. NARRATIVE by Leonard E. WHITE and Nell B. CANT Duke Institute for Brain Sciences Department of Neurobiology Duke University School of Medicine Overview When you view the lateral aspect of a human brain specimen (see Figures A3A and A102), three structures are usually visible: the cerebral hemispheres, the cerebellum, and part of the brainstem (although the brainstem is not visible in the specimen photographed in lateral view for Fig. 1 below). The spinal cord has usually been severed (but we’ll consider the spinal cord later), and the rest of the subdivisions are hidden from lateral view by the hemispheres. The diencephalon and the rest of the brainstem are visible on the medial surface of a brain that has been cut in the midsagittal plane. Parts of all of the subdivisions are also visible from the ventral surface of the whole brain. Over the next several tutorials, you will find video demonstrations (from the brain anatomy lab) and photographs (in the tutorial notes) of these brain surfaces, and sufficient detail in the narrative to appreciate the overall organization of the parts of the brain that are visible from each perspective. -
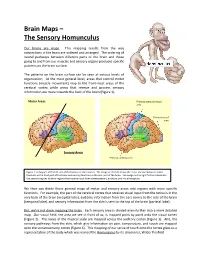
Brain Maps – the Sensory Homunculus
Brain Maps – The Sensory Homunculus Our brains are maps. This mapping results from the way connections in the brain are ordered and arranged. The ordering of neural pathways between different parts of the brain and those going to and from our muscles and sensory organs produces specific patterns on the brain surface. The patterns on the brain surface can be seen at various levels of organization. At the most general level, areas that control motor functions (muscle movement) map to the front-most areas of the cerebral cortex while areas that receive and process sensory information are more towards the back of the brain (Figure 1). Motor Areas Primary somatosensory area Primary visual area Sensory Areas Primary auditory area Figure 1. A diagram of the left side of the human cerebral cortex. The image on the left shows the major division between motor functions in the front part of the brain and sensory functions in the rear part of the brain. The image on the right further subdivides the sensory regions to show regions that receive input from somatosensory, auditory, and visual receptors. We then can divide these general maps of motor and sensory areas into regions with more specific functions. For example, the part of the cerebral cortex that receives visual input from the retina is in the very back of the brain (occipital lobe), auditory information from the ears comes to the side of the brain (temporal lobe), and sensory information from the skin is sent to the top of the brain (parietal lobe). But, we’re not done mapping the brain. -

Modulation of Interhemispheric Inhibition Between Primary Motor Cortices Induced by Manual Motor Imitation: a Transcranial Magnetic Stimulation Study
brain sciences Article Modulation of Interhemispheric Inhibition between Primary Motor Cortices Induced by Manual Motor Imitation: A Transcranial Magnetic Stimulation Study Dongting Tian 1,*, Shin-ichi Izumi 1,2 and Eizaburo Suzuki 1,3 1 Department of Physical Medicine and Rehabilitation, Tohoku University Graduate School of Medicine, Sendai 980-8575, Japan; [email protected] (S.-i.I.); [email protected] (E.S.) 2 Department of Physical Medicine and Rehabilitation, Tohoku University Graduate School of Biomedical Engineering, Sendai 980-8575, Japan 3 Department of Physical Therapy, Yamagata Prefectural University of Health Sciences, 260 Kamiyanagi, Yamagata 990-2212, Japan * Correspondence: [email protected] Abstract: Imitation has been proven effective in motor development and neurorehabilitation. How- ever, the relationship between imitation and interhemispheric inhibition (IHI) remains unclear. Transcranial magnetic stimulation (TMS) can be used to investigate IHI. In this study, the modifica- tion effects of IHI resulting from mirror neuron system (MNS) activation during different imitations are addressed. We measured IHI between homologous primary motor cortex (M1) by analyzing the ipsilateral silent period (iSP) evoked by single-pulse focal TMS during imitation and analyzed the respective IHI modulation during and after different patterns of imitation. Our main results showed that throughout anatomical imitation, significant time-course changes of iSP duration through the Citation: Tian, D.; Izumi, S.-i.; experiment were observed in both directions. iSP duration declined from the pre-imitation time Suzuki, E. Modulation of Interhemispheric Inhibition between point to the post-imitation time point and did not return to baseline after 30 min rest. -

Why Woodpecker Can Resist the Impact
Why woodpecker can resist the impact A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy Zhe Zhang Master of Civil Engineering, RMIT University, Melbourne, Australia School of Engineering College of Science, Engineering and Health RMIT University November 2019 II Declaration I certify that except where due acknowledgement has been made, the work is that of the author alone; the work has not been submitted previously, in whole or in part, to qualify for any other academic award; the content of the thesis is the result of work which has been carried out since the official commencement date of the approved research program; any editorial work, paid or unpaid, carried out by a third party is acknowledged; and ethics procedures and guidelines have been followed. Zhe Zhang 30 November 2019 III Acknowledgments The research in this thesis could not have been completed without significant support from many individuals and organisations. I would like to take this opportunity to express my deep gratitude to all of them. Firstly, I would like to express my sincere gratitude to my senior supervisor, Dr. Shiwei Zhou, for his wisdom in choosing this fascinating research topic for me and his constant encouragement and guidance. After the guidance of my Master graduation project, Dr. Zhou accepted me as one of the Ph.D. students and offered financial support for my first-year study at RMIT University. He has always been patient in providing guidance and offering supportive suggestions to me during my PhD candidature period. It is not an exaggeration to say that he has changed my life and his good characteristics have had a significant and positive impact on me which would be beneficial for the rest of my life. -

The Human Brain Hemisphere Controls the Left Side of the Body and the Left What Makes the Human Brain Unique Is Its Size
About the brain Cerebrum (also known as the The brain is made up of around 100 billion nerve cells - each one cerebral cortex or forebrain) is connected to another 10,000. This means that, in total, we The cerebrum is the largest part of the brain. It is split in to two have around 1,000 trillion connections in our brains. (This would ‘halves’ of roughly equal size called hemispheres. The two be written as 1,000,000,000,000,000). These are ultimately hemispheres, the left and right, are joined together by a bundle responsible for who we are. Our brains control the decisions we of nerve fibres called the corpus callosum. The right make, the way we learn, move, and how we feel. The human brain hemisphere controls the left side of the body and the left What makes the human brain unique is its size. Our brains have a hemisphere controls the right side of the body. The cerebrum is larger cerebral cortex, or cerebrum, relative to the rest of the The human brain is the centre of our nervous further divided in to four lobes: frontal, parietal, occipital, and brain than any other animal. (See the Cerebrum section of this temporal, which have different functions. system. It is the most complex organ in our fact sheet for further information.) This enables us to have abilities The frontal lobe body and is responsible for everything we do - such as complex language, problem-solving and self-control. The frontal lobe is located at the front of the brain.