In Vitro and in Vivo Trematode Models for Chemotherapeutic Studies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Literature Survey of Common Parasitic Zoonoses Encountered at Post-Mortem Examination in Slaughter Stocks in Tanzania: Economic and Public Health Implications
Volume 1- Issue 5 : 2017 DOI: 10.26717/BJSTR.2017.01.000419 Erick VG Komba. Biomed J Sci & Tech Res ISSN: 2574-1241 Research Article Open Access A Literature Survey of Common Parasitic Zoonoses Encountered at Post-Mortem Examination in Slaughter Stocks in Tanzania: Economic and Public Health Implications Erick VG Komba* Department of Veterinary Medicine and Public Health, Sokoine University of Agriculture, Tanzania Received: September 21, 2017; Published: October 06, 2017 *Corresponding author: Erick VG Komba, Senior lecturer, Department of Veterinary Medicine and Public Health, College of Veterinary Medicine and Biomedical Sciences, Sokoine University of Agriculture, P.O. Box 3021, Morogoro, Tanzania Abstract Zoonoses caused by parasites constitute a large group of infectious diseases with varying host ranges and patterns of transmission. Their public health impact of such zoonoses warrants appropriate surveillance to obtain enough information that will provide inputs in the design anddistribution, implementation prevalence of control and transmission strategies. Apatterns need therefore are affected arises by to the regularly influence re-evaluate of both human the current and environmental status of zoonotic factors. diseases, The economic particularly and in view of new data available as a result of surveillance activities and the application of new technologies. Consequently this paper summarizes available information in Tanzania on parasitic zoonoses encountered in slaughter stocks during post-mortem examination at slaughter facilities. The occurrence, in slaughter stocks, of fasciola spp, Echinococcus granulosus (hydatid) cysts, Taenia saginata Cysts, Taenia solium Cysts and ascaris spp. have been reported by various researchers. Information on these parasitic diseases is presented in this paper as they are the most important ones encountered in slaughter stocks in the country. -

The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca Volvulus Excretory Secretory Products
pathogens Review The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca volvulus Excretory Secretory Products Luc Vanhamme 1,*, Jacob Souopgui 1 , Stephen Ghogomu 2 and Ferdinand Ngale Njume 1,2 1 Department of Molecular Biology, Institute of Biology and Molecular Medicine, IBMM, Université Libre de Bruxelles, Rue des Professeurs Jeener et Brachet 12, 6041 Gosselies, Belgium; [email protected] (J.S.); [email protected] (F.N.N.) 2 Molecular and Cell Biology Laboratory, Biotechnology Unit, University of Buea, Buea P.O Box 63, Cameroon; [email protected] * Correspondence: [email protected] Received: 28 October 2020; Accepted: 18 November 2020; Published: 23 November 2020 Abstract: Nematodes constitute a very successful phylum, especially in terms of parasitism. Inside their mammalian hosts, parasitic nematodes mainly dwell in the digestive tract (geohelminths) or in the vascular system (filariae). One of their main characteristics is their long sojourn inside the body where they are accessible to the immune system. Several strategies are used by parasites in order to counteract the immune attacks. One of them is the expression of molecules interfering with the function of the immune system. Excretory-secretory products (ESPs) pertain to this category. This is, however, not their only biological function, as they seem also involved in other mechanisms such as pathogenicity or parasitic cycle (molting, for example). Wewill mainly focus on filariae ESPs with an emphasis on data available regarding Onchocerca volvulus, but we will also refer to a few relevant/illustrative examples related to other worm categories when necessary (geohelminth nematodes, trematodes or cestodes). -

Epidemiology of Human Fascioliasis
eserh ipidemiology of humn fsiolisisX review nd proposed new lssifition I P P wF F wsEgomD tFqF istenD 8 wFhF frgues he epidemiologil piture of humn fsiolisis hs hnged in reent yersF he numer of reports of humns psiol hepti hs inresed signifintly sine IWVH nd severl geogrphil res hve een infeted with desried s endemi for the disese in humnsD with prevlene nd intensity rnging from low to very highF righ prevlene of fsiolisis in humns does not neessrily our in res where fsiolisis is mjor veterinry prolemF rumn fsiolisis n no longer e onsidered merely s seondry zoonoti disese ut must e onsidered to e n importnt humn prsiti diseseF eordinglyD we present in this rtile proposed new lssifition for the epidemiology of humn fsiolisisF he following situtions re distinguishedX imported sesY utohthonousD isoltedD nononstnt sesY hypoED mesoED hyperED nd holoendemisY epidemis in res where fsiolisis is endemi in nimls ut not humnsY nd epidemis in humn endemi resF oir pge QRR le reÂsume en frnËisF in l p gin QRR figur un resumen en espnÄ olF ± severl rtiles report tht the inidene is sntrodution signifintly ggregted within fmily groups psiolisisD n infetion used y the liver fluke euse the individul memers hve shred the sme ontminted foodY psiol heptiD hs trditionlly een onsidered to e n importnt veterinry disese euse of the ± severl rtiles hve reported outreks not neessrily involving only fmily memersY nd sustntil prodution nd eonomi losses it uses in livestokD prtiulrly sheep nd ttleF sn ontrstD ± few rtiles hve reported epidemiologil surveys -

Coinfection of Schistosoma (Trematoda) with Bacteria, Protozoa and Helminths
CHAPTER 1 Coinfection of Schistosoma (Trematoda) with Bacteria, Protozoa and Helminths ,† ‡ Amy Abruzzi* and Bernard Fried Contents 1.1. Introduction 3 1.2. Coinfection of Species of Schistosoma and Plasmodium 4 1.2.1. Animal studies 21 1.2.2. Human studies 23 1.3. Coinfection of Schistosoma Species with Protozoans other than in the Genus Plasmodium 24 1.3.1. Leishmania 32 1.3.2. Toxoplasma 32 1.3.3. Entamoeba 34 1.3.4. Trypanosoma 35 1.4. Coinfection of Schistosoma Species with Salmonella 36 1.4.1. Animal studies 36 1.4.2. Human studies 42 1.5. Coinfection of Schistosoma Species with Bacteria other than Salmonella 43 1.5.1. Mycobacterium 43 1.5.2. Helicobacter pylori 49 1.5.3. Staphylococcus aureus 50 1.6. Coinfection of Schistosoma and Fasciola Species 50 1.6.1. Animal studies 57 1.6.2. Human studies 58 * Skillman Library, Lafayette College, Easton, Pennsylvania, USA { Epidemiology, University of Medicine and Dentistry of New Jersey (UMDNJ), Piscataway, New Jersey, USA { Department of Biology, Lafayette College, Easton, Pennsylvania, USA Advances in Parasitology, Volume 77 # 2011 Elsevier Ltd. ISSN 0065-308X, DOI: 10.1016/B978-0-12-391429-3.00005-8 All rights reserved. 1 2 Amy Abruzzi and Bernard Fried 1.7. Coinfection of Schistosoma Species and Helminths other than the Genus Fasciola 59 1.7.1. Echinostoma 59 1.7.2. Hookworm 70 1.7.3. Trichuris 70 1.7.4. Ascaris 71 1.7.5. Strongyloides and Trichostrongyloides 72 1.7.6. Filarids 73 1.8. Concluding Remarks 74 References 75 Abstract This review examines coinfection of selected species of Schisto- soma with bacteria, protozoa and helminths and focuses on the effects of the coinfection on the hosts. -
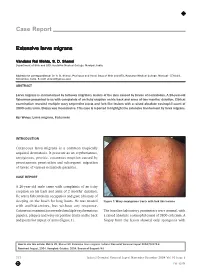
Extensive Larva Migrans
Case Report Extensive larva migrans Vandana Rai Mehta, S. D. Shenoi Department of Skin and STD, Kasturba Medical College, Manipal, India. Address for correspondence: Dr. S. D. Shenoi, Professor and Head, Dept of Skin and STD, Kasturba Medical College, Manipal - 576104, Karnataka, India. E-mail: [email protected] ABSTRACT Larva migrans is characterized by tortuous migratory lesions of the skin caused by larvae of nematodes. A 26-year-old fisherman presented to us with complaints of an itchy eruption on his back and arms of two months’ duration. Clinical examination revealed multiple wavy serpentine tracts and fork like lesions with a raised absolute eosinophil count of 3800 cells/cmm. Biopsy was inconclusive. This case is reported to highlight the extensive involvement by larva migrans. KEY WORDS: Larva migrans, Fisherman INTRODUCTION Cutaneous larva migrans is a common tropically acquired dermatosis. It presents as an erythematous, serpiginous, pruritic, cutaneous eruption caused by percutaneous penetration and subsequent migration of larvae of various nematode parasites. CASE REPORT A 26-year-old male came with complaints of an itchy eruption on his back and arms of 2 months’ duration. He was a fisherman by occupation and gave a history of sleeping on the beach for long hours. He was treated Figure 1: Wavy serpiginous tracts with fork like lesions with antihistamines, but without any response. Cutaneous examination revealed multiple erythematous The baseline laboratory parameters were normal, with papules, plaques and wavy serpentine tracts on the back a raised absolute eosinophil count of 3800 cell/cmm. A and posterior aspect of arms (Figure 1). biopsy from the lesion showed only spongiosis with How to cite this article: Mehta VR, Shenoi SD. -

Insecurities and Dogs: an Obstacle to the Eradication of Dracunculiasis
dicine & Me S l u a r ic g e p r o y r T ISSN: 2329-9088 Tropical Medicine & Surgery Review Article Insecurities and Dogs: An Obstacle to the Eradication of Dracunculiasis Aja Kalu1*,Nwufo Amanda2 Department of Care of the Elderly, Barking, Havering and Redbridge University Hospital, NHS Trust, Romford, Essex, UK; 2 Department of Public Health, University of Chester, Chester, UK. ABSTRACT Dracunculiasis is a parasitic worm infection also known as Guinea Worm Disease (GWD). It is caused by a nematode called Dracunculiasis Medinensis. It belongs to a group of communicable disease named Neglected Tropic Disease (NTD). Dracunculiasis is caused by drinking water contaminated with the vector copepods (water fleas). Although the disease is not fatal, the sores caused by the emerging worm in the lower limb can become secondarily infected and complications such as sepsis, tetanus can ensue. Also, the sores can cause abscess and cellulitis, leaving the individual incapacitated for weeks which extends beyond the emergence of the worm. Over the last three decades, the prevalence of Guinea worm disease has reduced drastically through cost effective intervention provided by The Cater Center, WHO, UNICEF with the disease targeted for eradication. Some African countries like Nigeria, Ghana, South Africa, and Kenya being the most recent, have eliminated the disease. Guinea worm is still present in Chad, Cameroon, Mali, Ethiopia where political instability, social inequalities and infection of dogs by the worm pose an increasing threat and obstacle to the elimination of the disease. Dracunculiasis represents a disease that can be eradicated without a drug or vaccine but with a cost-effective intervention that involves community efforts. -

The Effect of Triaenophorus Nodulosus (Cestoda: Bothriocephalidea) Infection on Some Biochemical Parameters of the Liver of Perca fluviatilis
J Parasit Dis (Oct-Dec 2019) 43(4):566–574 https://doi.org/10.1007/s12639-019-01128-0 ORIGINAL ARTICLE The effect of Triaenophorus nodulosus (Cestoda: Bothriocephalidea) infection on some biochemical parameters of the liver of Perca fluviatilis 1 1 1 Ekaterina V. Borvinskaya • Irina V. Sukhovskaya • Lev P. Smirnov • 1 1 1 Albina A. Kochneva • Aleksey N. Parshukov • Marina Yu. Krupnova • 1 1 1 Elizaveta A. Buoy • Rimma U. Vysotskaya • Maria V. Churova Received: 2 February 2019 / Accepted: 29 May 2019 / Published online: 5 June 2019 Ó Indian Society for Parasitology 2019 Abstract Natural infection of 2 to 6-year-old perch with Keywords Helminth Á Triaenophorus Á Cestoda Á the cestode parasites Triaenophorus nodulosus was shown Perca fluviatilis Á Invasion Á Biochemical status to have minor effects on the studied components of the antioxidant defense system, nucleic acids degradation, and carbohydrate metabolism enzymes in the liver of the fish. Introduction The level of infection of 1–4 parasite larvae per fish observed in wild population of perch was shown to be The study of the effect of parasites on the biochemical moderate in terms of its effect on the health of the host fish. status of their host is important for clarifying the mutual The activity of hepatic enzymes b-galactosidase, b-glu- adaptations in the parasite–host system. A parasite directly cosidase, cathepsin D, and glutathione S-transferase affects its host by competing with it for resources; never- showed different responses in infected males and females, theless, there is usually a balance in the system, where which indicates different potential resistance of fish to the parasites cannot cause major damage to the host popula- stress exposure between genders. -

Identification and Characterization of the Forkhead Box
IDENTIFICATION AND CHARACTERIZATION OF THE FORKHEAD BOX FAMILY OF TRANSCRIPTIONAL REGULATORS IN PARASITIC SCHISTOSOMES by MELISSA M. VARRECCHIA Submitted in partial fulfillment of the requirements for the degree of Doctor of philosophy Department of Biology CASE WESTERN RESERVE UNIVERSITY August 2017 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the dissertation of Melissa M. Varrecchia candidate for the degree of Doctor of Philosophy Committee Chair Michael F. Benard Committee Member Emmitt R. Jolly Committee Member Christopher A. Cullis Committee Member Claudia M. Mizutani Committee Member Brian M. McDermott Date of Defense June 6, 2017 *We also certify that written approval has been obtained for any proprietary material contained therein. ii Dedication I would like to dedicate this dissertation to my Mom and Dad. Mom, thank you for your endless love, support and encouragement throughout the years. Dad, I miss you and I know that you are with me always, cheering me on in spirit. iii Table of Contents Table of Contents………………………………………………………………………...1 List of Tables……………………………………………………………………………..6 List of Figures…………………………………………………………………………....8 Acknowledgements…………………………………………………………………..…11 List of Abbreviations…………………………………………………………………...13 Abstract…………………………………………………………………………………15 Chapter 1: Introduction………………………………………………………………..17 1.1 Schistosomiasis………………………………………………………………17 1.2 Pathogenesis and treatment…………………………………………………..18 1.3 Schistosome life cycle………………………………………………………..20 1.4 Schistosome morphology -

Waterborne Zoonotic Helminthiases Suwannee Nithiuthaia,*, Malinee T
Veterinary Parasitology 126 (2004) 167–193 www.elsevier.com/locate/vetpar Review Waterborne zoonotic helminthiases Suwannee Nithiuthaia,*, Malinee T. Anantaphrutib, Jitra Waikagulb, Alvin Gajadharc aDepartment of Pathology, Faculty of Veterinary Science, Chulalongkorn University, Henri Dunant Road, Patumwan, Bangkok 10330, Thailand bDepartment of Helminthology, Faculty of Tropical Medicine, Mahidol University, Ratchawithi Road, Bangkok 10400, Thailand cCentre for Animal Parasitology, Canadian Food Inspection Agency, Saskatoon Laboratory, Saskatoon, Sask., Canada S7N 2R3 Abstract This review deals with waterborne zoonotic helminths, many of which are opportunistic parasites spreading directly from animals to man or man to animals through water that is either ingested or that contains forms capable of skin penetration. Disease severity ranges from being rapidly fatal to low- grade chronic infections that may be asymptomatic for many years. The most significant zoonotic waterborne helminthic diseases are either snail-mediated, copepod-mediated or transmitted by faecal-contaminated water. Snail-mediated helminthiases described here are caused by digenetic trematodes that undergo complex life cycles involving various species of aquatic snails. These diseases include schistosomiasis, cercarial dermatitis, fascioliasis and fasciolopsiasis. The primary copepod-mediated helminthiases are sparganosis, gnathostomiasis and dracunculiasis, and the major faecal-contaminated water helminthiases are cysticercosis, hydatid disease and larva migrans. Generally, only parasites whose infective stages can be transmitted directly by water are discussed in this article. Although many do not require a water environment in which to complete their life cycle, their infective stages can certainly be distributed and acquired directly through water. Transmission via the external environment is necessary for many helminth parasites, with water and faecal contamination being important considerations. -

Public Health Significance of Intestinal Parasitic Infections*
Articles in the Update series Les articles de la rubrique give a concise, authoritative, Le pointfournissent un bilan and up-to-date survey of concis et fiable de la situa- the present position in the tion actuelle dans les do- Update selectedfields, coveringmany maines consideres, couvrant different aspects of the de nombreux aspects des biomedical sciences and sciences biomedicales et de la , po n t , , public health. Most of santepublique. Laplupartde the articles are written by ces articles auront donc ete acknowledged experts on the redigeis par les specialistes subject. les plus autorises. Bulletin of the World Health Organization, 65 (5): 575-588 (1987) © World Health Organization 1987 Public health significance of intestinal parasitic infections* WHO EXPERT COMMITTEE' Intestinal parasitic infections are distributed virtually throughout the world, with high prevalence rates in many regions. Amoebiasis, ascariasis, hookworm infection and trichuriasis are among the ten most common infections in the world. Other parasitic infections such as abdominal angiostrongyliasis, intestinal capil- lariasis, and strongyloidiasis are of local or regional public health concern. The prevention and control of these infections are now more feasible than ever before owing to the discovery of safe and efficacious drugs, the improvement and sim- plification of some diagnostic procedures, and advances in parasite population biology. METHODS OF ASSESSMENT The amount of harm caused by intestinal parasitic infections to the health and welfare of individuals and communities depends on: (a) the parasite species; (b) the intensity and course of the infection; (c) the nature of the interactions between the parasite species and concurrent infections; (d) the nutritional and immunological status of the population; and (e) numerous socioeconomic factors. -

Risk of Soil-Transmitted Helminthiasis Among Agrarian Communities of Kogi
bioRxiv preprint doi: https://doi.org/10.1101/663237; this version posted June 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. 1 1 Long title: 2 Risk of soil-transmitted helminthiasis among agrarian communities of Kogi 3 State, Nigeria: Evaluated in the context of The Soil-Transmitted 4 Helminthiasis Advisory Committee recommendation 2016 5 Short title: 6 Soil-transmitted helminthiasis in Kogi State 7 8 Joy T. Anunobi1, Ikem C. Okoye2*, Ifeanyi Oscar N. Aguzie2*, Yvonne E. Ndukwe2 and 9 Onyekachi J. Okpasuo2 10 1 Science Laboratory Technology Department, Federal Polytechnic, Idah, Kogi State, Nigeria. 11 2Parasitology and Public Health Unit, Department of Zoology and Environmental Biology, 12 University of Nigeria, Nsukka, Enugu State, Nigeria. 13 *Corresponding authors: 14 E-mail: [email protected] (IONA) 15 E-mail: [email protected] (ICO) 16 Authors’ contributions 17 Conceptualization and Methodology: Joy T. Anunobi and Ikem C. Okoye 18 Investigation: Joy T. Anunobi, Yvonne E. Ndukwe and Onyekachi J. Okpasuo 19 Data curation, formal analysis & software: Ifeanyi Oscar N. Aguzie 20 Writing of first draft: Joy T. Anunobi and Ifeanyi Oscar N. Aguzie 21 Supervision: Ikem C. Okoye 22 Final draft: All the authors approved the final draft. 23 Funding 24 The study did not receive external funding 1 bioRxiv preprint doi: https://doi.org/10.1101/663237; this version posted June 7, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. -

Federal Register/Vol. 85, No. 136/Wednesday, July 15, 2020
Federal Register / Vol. 85, No. 136 / Wednesday, July 15, 2020 / Notices 42883 interest to the IRS product DEPARTMENT OF HEALTH AND SUPPLEMENTARY INFORMATION: manufacturers who submitted timely HUMAN SERVICES Table of Contents exceptions, to determine whether the companies remained interested in Food and Drug Administration I. Background: Priority Review Voucher pursuing their appeals of the ALJ’s Program [Docket No. FDA–2008–N–0567] II. Diseases Being Designated Initial Decision. FDA informed the A. Opisthorchiasis companies that, if they did not respond Designating Additions to the Current B. Paragonimiasis and affirm their desire to pursue their List of Tropical Diseases in the Federal III. Process for Requesting Additional appeals by January 8, 2018, the Office of Food, Drug, and Cosmetic Act Diseases To Be Added to the List the Commissioner would conclude that IV. Paperwork Reduction Act AGENCY: Food and Drug Administration, V. References the companies no longer wish to pursue HHS. the appeal of the ALJ’s Initial Decision ACTION: Final order. I. Background: Priority Review and will proceed as if the appeals have Voucher Program been withdrawn. The Office of the SUMMARY: The Federal Food, Drug, and Section 524 of the FD&C Act (21 Commissioner did not receive a Cosmetic Act (FD&C Act) authorizes the U.S.C. 360n), which was added by response from any of the companies by Food and Drug Administration (FDA or section 1102 of the Food and Drug the given date; therefore, the Agency) to award priority review Administration Amendments Act of Commissioner now deems the vouchers (PRVs) to tropical disease 2007 (Pub.