Risk of Soil-Transmitted Helminthiasis Among Agrarian Communities of Kogi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca Volvulus Excretory Secretory Products
pathogens Review The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca volvulus Excretory Secretory Products Luc Vanhamme 1,*, Jacob Souopgui 1 , Stephen Ghogomu 2 and Ferdinand Ngale Njume 1,2 1 Department of Molecular Biology, Institute of Biology and Molecular Medicine, IBMM, Université Libre de Bruxelles, Rue des Professeurs Jeener et Brachet 12, 6041 Gosselies, Belgium; [email protected] (J.S.); [email protected] (F.N.N.) 2 Molecular and Cell Biology Laboratory, Biotechnology Unit, University of Buea, Buea P.O Box 63, Cameroon; [email protected] * Correspondence: [email protected] Received: 28 October 2020; Accepted: 18 November 2020; Published: 23 November 2020 Abstract: Nematodes constitute a very successful phylum, especially in terms of parasitism. Inside their mammalian hosts, parasitic nematodes mainly dwell in the digestive tract (geohelminths) or in the vascular system (filariae). One of their main characteristics is their long sojourn inside the body where they are accessible to the immune system. Several strategies are used by parasites in order to counteract the immune attacks. One of them is the expression of molecules interfering with the function of the immune system. Excretory-secretory products (ESPs) pertain to this category. This is, however, not their only biological function, as they seem also involved in other mechanisms such as pathogenicity or parasitic cycle (molting, for example). Wewill mainly focus on filariae ESPs with an emphasis on data available regarding Onchocerca volvulus, but we will also refer to a few relevant/illustrative examples related to other worm categories when necessary (geohelminth nematodes, trematodes or cestodes). -
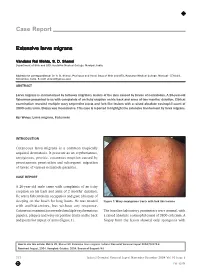
Extensive Larva Migrans
Case Report Extensive larva migrans Vandana Rai Mehta, S. D. Shenoi Department of Skin and STD, Kasturba Medical College, Manipal, India. Address for correspondence: Dr. S. D. Shenoi, Professor and Head, Dept of Skin and STD, Kasturba Medical College, Manipal - 576104, Karnataka, India. E-mail: [email protected] ABSTRACT Larva migrans is characterized by tortuous migratory lesions of the skin caused by larvae of nematodes. A 26-year-old fisherman presented to us with complaints of an itchy eruption on his back and arms of two months’ duration. Clinical examination revealed multiple wavy serpentine tracts and fork like lesions with a raised absolute eosinophil count of 3800 cells/cmm. Biopsy was inconclusive. This case is reported to highlight the extensive involvement by larva migrans. KEY WORDS: Larva migrans, Fisherman INTRODUCTION Cutaneous larva migrans is a common tropically acquired dermatosis. It presents as an erythematous, serpiginous, pruritic, cutaneous eruption caused by percutaneous penetration and subsequent migration of larvae of various nematode parasites. CASE REPORT A 26-year-old male came with complaints of an itchy eruption on his back and arms of 2 months’ duration. He was a fisherman by occupation and gave a history of sleeping on the beach for long hours. He was treated Figure 1: Wavy serpiginous tracts with fork like lesions with antihistamines, but without any response. Cutaneous examination revealed multiple erythematous The baseline laboratory parameters were normal, with papules, plaques and wavy serpentine tracts on the back a raised absolute eosinophil count of 3800 cell/cmm. A and posterior aspect of arms (Figure 1). biopsy from the lesion showed only spongiosis with How to cite this article: Mehta VR, Shenoi SD. -

Insecurities and Dogs: an Obstacle to the Eradication of Dracunculiasis
dicine & Me S l u a r ic g e p r o y r T ISSN: 2329-9088 Tropical Medicine & Surgery Review Article Insecurities and Dogs: An Obstacle to the Eradication of Dracunculiasis Aja Kalu1*,Nwufo Amanda2 Department of Care of the Elderly, Barking, Havering and Redbridge University Hospital, NHS Trust, Romford, Essex, UK; 2 Department of Public Health, University of Chester, Chester, UK. ABSTRACT Dracunculiasis is a parasitic worm infection also known as Guinea Worm Disease (GWD). It is caused by a nematode called Dracunculiasis Medinensis. It belongs to a group of communicable disease named Neglected Tropic Disease (NTD). Dracunculiasis is caused by drinking water contaminated with the vector copepods (water fleas). Although the disease is not fatal, the sores caused by the emerging worm in the lower limb can become secondarily infected and complications such as sepsis, tetanus can ensue. Also, the sores can cause abscess and cellulitis, leaving the individual incapacitated for weeks which extends beyond the emergence of the worm. Over the last three decades, the prevalence of Guinea worm disease has reduced drastically through cost effective intervention provided by The Cater Center, WHO, UNICEF with the disease targeted for eradication. Some African countries like Nigeria, Ghana, South Africa, and Kenya being the most recent, have eliminated the disease. Guinea worm is still present in Chad, Cameroon, Mali, Ethiopia where political instability, social inequalities and infection of dogs by the worm pose an increasing threat and obstacle to the elimination of the disease. Dracunculiasis represents a disease that can be eradicated without a drug or vaccine but with a cost-effective intervention that involves community efforts. -

Identification and Characterization of the Forkhead Box
IDENTIFICATION AND CHARACTERIZATION OF THE FORKHEAD BOX FAMILY OF TRANSCRIPTIONAL REGULATORS IN PARASITIC SCHISTOSOMES by MELISSA M. VARRECCHIA Submitted in partial fulfillment of the requirements for the degree of Doctor of philosophy Department of Biology CASE WESTERN RESERVE UNIVERSITY August 2017 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the dissertation of Melissa M. Varrecchia candidate for the degree of Doctor of Philosophy Committee Chair Michael F. Benard Committee Member Emmitt R. Jolly Committee Member Christopher A. Cullis Committee Member Claudia M. Mizutani Committee Member Brian M. McDermott Date of Defense June 6, 2017 *We also certify that written approval has been obtained for any proprietary material contained therein. ii Dedication I would like to dedicate this dissertation to my Mom and Dad. Mom, thank you for your endless love, support and encouragement throughout the years. Dad, I miss you and I know that you are with me always, cheering me on in spirit. iii Table of Contents Table of Contents………………………………………………………………………...1 List of Tables……………………………………………………………………………..6 List of Figures…………………………………………………………………………....8 Acknowledgements…………………………………………………………………..…11 List of Abbreviations…………………………………………………………………...13 Abstract…………………………………………………………………………………15 Chapter 1: Introduction………………………………………………………………..17 1.1 Schistosomiasis………………………………………………………………17 1.2 Pathogenesis and treatment…………………………………………………..18 1.3 Schistosome life cycle………………………………………………………..20 1.4 Schistosome morphology -

Public Health Significance of Intestinal Parasitic Infections*
Articles in the Update series Les articles de la rubrique give a concise, authoritative, Le pointfournissent un bilan and up-to-date survey of concis et fiable de la situa- the present position in the tion actuelle dans les do- Update selectedfields, coveringmany maines consideres, couvrant different aspects of the de nombreux aspects des biomedical sciences and sciences biomedicales et de la , po n t , , public health. Most of santepublique. Laplupartde the articles are written by ces articles auront donc ete acknowledged experts on the redigeis par les specialistes subject. les plus autorises. Bulletin of the World Health Organization, 65 (5): 575-588 (1987) © World Health Organization 1987 Public health significance of intestinal parasitic infections* WHO EXPERT COMMITTEE' Intestinal parasitic infections are distributed virtually throughout the world, with high prevalence rates in many regions. Amoebiasis, ascariasis, hookworm infection and trichuriasis are among the ten most common infections in the world. Other parasitic infections such as abdominal angiostrongyliasis, intestinal capil- lariasis, and strongyloidiasis are of local or regional public health concern. The prevention and control of these infections are now more feasible than ever before owing to the discovery of safe and efficacious drugs, the improvement and sim- plification of some diagnostic procedures, and advances in parasite population biology. METHODS OF ASSESSMENT The amount of harm caused by intestinal parasitic infections to the health and welfare of individuals and communities depends on: (a) the parasite species; (b) the intensity and course of the infection; (c) the nature of the interactions between the parasite species and concurrent infections; (d) the nutritional and immunological status of the population; and (e) numerous socioeconomic factors. -

In Vitro and in Vivo Trematode Models for Chemotherapeutic Studies
589 In vitro and in vivo trematode models for chemotherapeutic studies J. KEISER* Department of Medical Parasitology and Infection Biology, Swiss Tropical Institute, CH-4002 Basel, Switzerland (Received 27 June 2009; revised 7 August 2009 and 26 October 2009; accepted 27 October 2009; first published online 7 December 2009) SUMMARY Schistosomiasis and food-borne trematodiases are chronic parasitic diseases affecting millions of people mostly in the developing world. Additional drugs should be developed as only few drugs are available for treatment and drug resistance might emerge. In vitro and in vivo whole parasite screens represent essential components of the trematodicidal drug discovery cascade. This review describes the current state-of-the-art of in vitro and in vivo screening systems of the blood fluke Schistosoma mansoni, the liver fluke Fasciola hepatica and the intestinal fluke Echinostoma caproni. Examples of in vitro and in vivo evaluation of compounds for activity are presented. To boost the discovery pipeline for these diseases there is a need to develop validated, robust high-throughput in vitro systems with simple readouts. Key words: Schistosoma mansoni, Fasciola hepatica, Echinostoma caproni, in vitro, in vivo, drug discovery, chemotherapy. INTRODUCTION by chemotherapy. However, only two drugs are currently available: triclabendazole against fascio- Thus far approximately 6000 species in the sub-class liasis and praziquantel against the other food-borne Digenea, phylum Platyhelminthes have been de- trematode infections and schistosomiasis (Keiser and scribed in the literature. Among them, only a dozen Utzinger, 2004; Keiser et al. 2005). Hence, there is a or so species parasitize humans. These include need for discovery and development of new drugs, the blood flukes (five species of Schistosoma), liver particularly in view of growing concern about re- flukes (Clonorchis sinensis, Fasciola gigantica, Fasciola sistance developing to existing drugs. -

Progress Toward Global Eradication of Dracunculiasis — January 2012–June 2013
Morbidity and Mortality Weekly Report Weekly / Vol. 62 / No. 42 October 25, 2013 Progress Toward Global Eradication of Dracunculiasis — January 2012–June 2013 Dracunculiasis (Guinea worm disease) is caused by water from bore-hole or hand-dug wells (6). Containment of Dracunculus medinensis, a parasitic worm. Approximately transmission,* achieved through 1) voluntary isolation of each 1 year after infection from contaminated drinking water, the patient to prevent contamination of drinking water sources, worm emerges through the skin of the infected person, usually 2) provision of first aid, 3) manual extraction of the worm, on the lower limb. Pain and secondary bacterial infection can and 4) application of occlusive bandages, complements the cause temporary or permanent disability that disrupts work four main interventions. and schooling. In 1986, the World Health Assembly (WHA) Countries enter the WHO precertification stage of eradica- called for dracunculiasis elimination (1), and the global tion after completing 1 full calendar year without reporting any Guinea Worm Eradication Program, supported by The Carter indigenous cases (i.e., one incubation period for D. medinensis). Center, World Health Organization (WHO), United Nations A case of dracunculiasis is defined as infection occurring in Children’s Fund (UNICEF), CDC, and other partners, began * Transmission from a patient with dracunculiasis is contained if all of the assisting ministries of health of dracunculiasis-endemic coun- following conditions are met: 1) the disease is detected <24 hours after worm tries in meeting this goal. At that time, an estimated 3.5 million emergence; 2) the patient has not entered any water source since the worm cases occurred each year in 20 countries in Africa and Asia emerged; 3) a volunteer has managed the patient properly, by cleaning and bandaging the lesion until the worm has been fully removed manually and by (1,2). -

Transmitted Helminth Infections in Chinese Schoolchildren
Impact of a video-based health education intervention on soil- transmitted helminth infections in Chinese schoolchildren Franziska Andrea Bieri MSc, BSc A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2013 School of Population Health Abstract Worldwide, more than 2 billion people are infected with soil-transmitted helminths (STH), commonly known as intestinal worms. The STH are a group of parasitic nematodes which include roundworms (Ascaris lumbricoides), whipworms (Trichuris trichiura) and hookworms (Necator americanus and Ancylostoma duodenale). Diseases caused by the STH belong to the group of Neglected Tropical Diseases, comprising 17 infections currently targeted for prevention and research by the World Health Organization, the United Nations Development Program and the World Bank. STH are intimately connected with rural poverty, inadequate sanitation and waste disposal, lack of clean water and poor hygiene, as well as limited access to health care and preventive measures such as health education. Even though STH rarely cause death, they impact significantly on public health and cause severe disability in the world’s poorest countries. The worldwide burden of STH has been estimated to be as high as 39 million disability-adjusted life years (DALYs), with almost half of the global disease burden due to these worm infections afflicting children aged 5-14 years. Nevertheless, STH remain largely neglected by the medical and international community. In China, STH still impact substantially on public health with an estimated 129 million infected people and children aged 5-14 years having the highest rates of infection. Major endemic foci are observed in the central, western and southern provinces. -

Zoonotic Soil-Transmitted Helminths: a Triad of Worms, Animals, and Humans and the Veterinarian’S Role in Community Public Health
Master of Public Health Field Experience Report ZOONOTIC SOIL-TRANSMITTED HELMINTHS: A TRIAD OF WORMS, ANIMALS, AND HUMANS AND THE VETERINARIAN’S ROLE IN COMMUNITY PUBLIC HEALTH by DEBORAH K. GLAUM, D.V.M. MPH Candidate submitted in partial fulfillment of the requirements for the degree MASTER OF PUBLIC HEALTH Graduate Committee: Patricia A. Payne, BS, DVM, PhD Justin J. Kastner, BS, MSc, PgDip, PhD Abbey Nutsch, BS, PhD Field Experience Site: Lawrence/Douglas County Health Department, Lawrence, KS June to December 2013 Field Experience Preceptor: Charles Bryan, BS, MPA KANSAS STATE UNIVERSITY Manhattan, Kansas 2015 i Copyright DEBORAH KAY GLAUM, D.V.M. 2015 ii Abstract Deborah K. Glaum, DVM Dr. Patricia Payne Kansas State University, 2015 Major Professor Although mankind has known about intestinal parasites for millennia, it has only been in the last century that scientists have recognized the diseases caused by zoonotic parasites. Soil- transmitted helminths (STHs), including Toxocara canis and Ancylostoma caninum, are an important subset of zoonotic parasites, and they have developed unique characteristics that allow them to infect humans and animals. Overt parasitism is no longer common in many developed nations; however, the subtle and varied symptoms associated with STH infection, along with the lack of experience and interest of the human healthcare industry, mean that many cases of zoonotic infection are missed. These diseases not only affect the health of humans, but they also have poverty-promoting effects. The lack of attention paid to these parasites in recent years has caused the World Health Organization to label them “neglected zoonotic diseases.” Human culture and individual behaviors are important risk factors for these diseases, as well as climate and geography. -

Lymphatic Filariasis
BiologyPUBLIC HEALTH IMPORTANCE 29 In areas with low or moderate transmission of malaria, in those with advanced health services with well trained and experienced personnel, and in priority areas such as those with development projects, attempts may be made to reduce the prevalence of malaria by community-wide mosquito control measures. In areas subject to epidemic risk, quick-acting and timely vector control mea- sures, such as insecticide spraying, play an important role in the control or prevention of epidemics. Apart from the input of health services in the planning and management of activities, it is also important for communities to participate in control efforts. Sufficient resources have to be ensured for the long-term maintenance of improve- ments obtained. In developed countries with advanced professional capabilities and sufficient resources, it is possible to aim at a countrywide eradication of malaria. Eradication has been achieved in southern Europe, most Caribbean islands, the Maldives, large parts of the former USSR and the USA. As most anopheline mosquitos enter houses to bite and rest, malaria control programmes have focused primarily on the indoor application of residual insecti- cides to the walls and ceilings of houses. House spraying is still important in some tropical countries but in others its significance is diminishing because of a number of problems (see Chapter 9), which, in certain areas, have led to the interruption or termination of malaria control programmes. There has been increased interest in other control methods that would avoid some of the problems related to house spraying. Methods that are less costly and easier to organize, such as community-wide use of impregnated bednets, and methods that bring about long- lasting or permanent improvements by eliminating breeding places are now being increasingly considered. -

Zoonotic Implications of Onchocerca Species on Human Health
pathogens Review Zoonotic Implications of Onchocerca Species on Human Health Maria Cambra-Pellejà 1,2, Javier Gandasegui 3 , Rafael Balaña-Fouce 4 , José Muñoz 3 and María Martínez-Valladares 1,2,* 1 Instituto de Ganadería de Montaña (CSIC-Universidad de León), 24346 León, Spain; [email protected] 2 Departamento de Sanidad Animal, Facultad de Veterinaria, Universidad de León, Campus de Vegazana, 24071 León, Spain 3 Instituto de Salud Global de Barcelona (ISGlobal), 08036 Barcelona, Spain; [email protected] (J.G.); [email protected] (J.M.) 4 Departmento de Ciencias Biomédicas, Facultad de Veterinaria, Universidad de León, 24071 León, Spain; [email protected] * Correspondence: [email protected] Received: 31 July 2020; Accepted: 15 September 2020; Published: 17 September 2020 Abstract: The genus Onchocerca includes several species associated with ungulates as hosts, although some have been identified in canids, felids, and humans. Onchocerca species have a wide geographical distribution, and the disease they produce, onchocerciasis, is generally seen in adult individuals because of its large prepatency period. In recent years, Onchocerca species infecting animals have been found as subcutaneous nodules or invading the ocular tissues of humans; the species involved are O. lupi, O. dewittei japonica, O. jakutensis, O. gutturosa, and O. cervicalis. These findings generally involve immature adult female worms, with no evidence of being fertile. However, a few cases with fertile O. lupi, O. dewittei japonica, and O. jakutensis worms have been identified recently in humans. These are relevant because they indicate that the parasite’s life cycle was completed in the new host—humans. In this work, we discuss the establishment of zoonotic Onchocerca infections in humans, and the possibility of these infections to produce symptoms similar to human onchocerciasis, such as dermatitis, ocular damage, and epilepsy. -

Anisakiosis and Pseudoterranovosis
National Wildlife Health Center Anisakiosis and Pseudoterranovosis Circular 1393 U.S. Department of the Interior U.S. Geological Survey 1 2 3 4 5 6 7 8 Cover: 1. Common seal, by Andreas Trepte, CC BY-SA 2.5; 2. Herring catch, National Oceanic and Atmospheric Administration; 3. Larval Pseudoterranova sp. in muscle of an American plaice, by Dr. Lena Measures; 4. Salmon sashimi, by Blu3d, Lilyu, GFDL CC BY-SA3.0; 5. Beluga whale, by Jofre Ferrer, CC BY-NC-ND 2.0; 6. Dolphin, National Aeronautics and Space Administration; 7. Squid, National Oceanic and Atmospheric Administration; 8. Krill, by Oystein Paulsen, CC BY-SA 3.0. Anisakiosis and Pseudoterranovosis By Lena N. Measures Edited by Rachel C. Abbott and Charles van Riper, III USGS National Wildlife Health Center Circular 1393 U.S. Department of the Interior U.S. Geological Survey ii U.S. Department of the Interior SALLY JEWELL, Secretary U.S. Geological Survey Suzette M. Kimball, Acting Director U.S. Geological Survey, Reston, Virginia: 2014 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit http://www.usgs.gov or call 1–888–ASK–USGS For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Although this information product, for the most part, is in the public domain, it also may contain copyrighted materials as noted in the text.