Zoonotic Implications of Onchocerca Species on Human Health
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca Volvulus Excretory Secretory Products
pathogens Review The Functional Parasitic Worm Secretome: Mapping the Place of Onchocerca volvulus Excretory Secretory Products Luc Vanhamme 1,*, Jacob Souopgui 1 , Stephen Ghogomu 2 and Ferdinand Ngale Njume 1,2 1 Department of Molecular Biology, Institute of Biology and Molecular Medicine, IBMM, Université Libre de Bruxelles, Rue des Professeurs Jeener et Brachet 12, 6041 Gosselies, Belgium; [email protected] (J.S.); [email protected] (F.N.N.) 2 Molecular and Cell Biology Laboratory, Biotechnology Unit, University of Buea, Buea P.O Box 63, Cameroon; [email protected] * Correspondence: [email protected] Received: 28 October 2020; Accepted: 18 November 2020; Published: 23 November 2020 Abstract: Nematodes constitute a very successful phylum, especially in terms of parasitism. Inside their mammalian hosts, parasitic nematodes mainly dwell in the digestive tract (geohelminths) or in the vascular system (filariae). One of their main characteristics is their long sojourn inside the body where they are accessible to the immune system. Several strategies are used by parasites in order to counteract the immune attacks. One of them is the expression of molecules interfering with the function of the immune system. Excretory-secretory products (ESPs) pertain to this category. This is, however, not their only biological function, as they seem also involved in other mechanisms such as pathogenicity or parasitic cycle (molting, for example). Wewill mainly focus on filariae ESPs with an emphasis on data available regarding Onchocerca volvulus, but we will also refer to a few relevant/illustrative examples related to other worm categories when necessary (geohelminth nematodes, trematodes or cestodes). -

Trichinosis (Trichinellosis) Case Reporting and Investigation Protocol
Wisconsin Department of Health Services Division of Public Health P-01912 (Rev 08/2017) Communicable Disease Case Reporting and Investigation Protocol TRICHINOSIS (TRICHINELLOSIS) I. IDENTIFICATION AND DEFINITION OF CASES A. Clinical Description: A parasitic disease caused by ingestion of Trichinella species larvae. The disease causes a variety of clinical manifestations. Common signs and symptoms among symptomatic persons include eosinophilia, fever, myalgia, and periorbital edema. B. Laboratory Criteria: Confirmatory laboratory evidence: • Demonstration of Trichinella larvae on muscle biopsy, OR • A positive serology for Trichinella. C. Wisconsin Surveillance Case Definition: A clinically compatible illness that is laboratory confirmed. NOTE: In an outbreak setting, at least one case must be laboratory confirmed. Associated cases are considered confirmed if the patient shared an epidemiologically implicated meal or ate an epidemiologically implicated meat product and has either a positive serology for trichinosis or a clinically compatible illness. II. REPORTING A. Wisconsin Disease Surveillance Category II – Methods for Reporting: This disease shall be reported to the patient’s local health officer or to the local health officer’s designee within 72 hours of recognition of a case or suspected case, per Wis. Admin. Code § DHS 145.04 (3) (b). Report electronically through the Wisconsin Electronic Disease Surveillance System (WEDSS), or mail or fax a completed Acute and Communicable Disease Case Report (F-44151) to the address on the form. B. Responsibility for Reporting: According to Wis. Admin. Code § DHS 145.04(1), persons licensed under Wis. Stat. ch. 441 or 448, laboratories, health care facilities, teachers, principals, or nurses serving a school or day care center, and any person who knows or suspects that a person has a communicable disease identified in Appendix A. -

Onchocerciasis
11 ONCHOCERCIASIS ADRIAN HOPKINS AND BOAKYE A. BOATIN 11.1 INTRODUCTION the infection is actually much reduced and elimination of transmission in some areas has been achieved. Differences Onchocerciasis (or river blindness) is a parasitic disease in the vectors in different regions of Africa, and differences in cause by the filarial worm, Onchocerca volvulus. Man is the the parasite between its savannah and forest forms led to only known animal reservoir. The vector is a small black fly different presentations of the disease in different areas. of the Simulium species. The black fly breeds in well- It is probable that the disease in the Americas was brought oxygenated water and is therefore mostly associated with across from Africa by infected people during the slave trade rivers where there is fast-flowing water, broken up by catar- and found different Simulium flies, but ones still able to acts or vegetation. All populations are exposed if they live transmit the disease (3). Around 500,000 people were at risk near the breeding sites and the clinical signs of the disease in the Americas in 13 different foci, although the disease has are related to the amount of exposure and the length of time recently been eliminated from some of these foci, and there is the population is exposed. In areas of high prevalence first an ambitious target of eliminating the transmission of the signs are in the skin, with chronic itching leading to infection disease in the Americas by 2012. and chronic skin changes. Blindness begins slowly with Host factors may also play a major role in the severe skin increasingly impaired vision often leading to total loss of form of the disease called Sowda, which is found mostly in vision in young adults, in their early thirties, when they northern Sudan and in Yemen. -

STUDY of PARASITIC INFESTATION and ITS EFFECT on the HEALTH STATUS of PRIMARY SCHOOL CHILDREN in TANTA CITY Nour Abd El Azize Mohammed Mealy, Prof
STUDY OF PARASITIC INFESTATION AND ITS EFFECT ON THE HEALTH STATUS OF PRIMARY SCHOOL CHILDREN IN TANTA CITY Nour Abd El Azize Mohammed Mealy, Prof. Dr. Nadia Yahia Ismaiel, Prof. Dr. Hassan Saad Abu Saif, Prof. Dr. Wael Refaat Hablas STUDY OF PARASITIC INFESTATION AND ITS EFFECT ON THE HEALTH STATUS OF PRIMARY SCHOOL CHILDREN IN TANTA CITY By Nour Abd El Azize Mohammed Mealy, Prof. Dr. Nadia Yahia Ismaiel*, Prof. Dr. Hassan Saad Abu Saif*, Prof. Dr. Wael Refaat Hablas** Pediatric*& Clinical Pathology** Depts. Al-Azhar University- Faculty of Medicine ABSTRACT Background: School age children are one of the groups at high-risk for intestinal parasitic infestations. Factors like poor developments of hygienic habits, immune system and over-crowding contributes for infestation. The adverse effects of intestinal parasites among children are diverse and alarming. Intestinal parasitic infestations have detrimental effects on the survival, appetite, growth and physical fitness, school attendance and cognitive performance of school age children (Alemu et al., 2011). Objectives: We aimed to 1. Assess the prevalence of parasitic infestation and its effect on the health status of primary school children in Tanta City (5 schools from 3 areas at Tanta city) 2. Determine the prevalence of intestinal parasitic infestation among primary school children in some urban communities of Tanta City 3. Identify associated risk factors of school children for parasitic infestations in some urban communities of Tanta City. Design: This is descriptive cross sectional study that was carried out on 1000 students (boys &girls) at governmental primary schools at Tanta rural areas. This research was continued until fulfillment of the study from April 2017 to May 2018. -
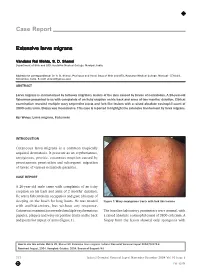
Extensive Larva Migrans
Case Report Extensive larva migrans Vandana Rai Mehta, S. D. Shenoi Department of Skin and STD, Kasturba Medical College, Manipal, India. Address for correspondence: Dr. S. D. Shenoi, Professor and Head, Dept of Skin and STD, Kasturba Medical College, Manipal - 576104, Karnataka, India. E-mail: [email protected] ABSTRACT Larva migrans is characterized by tortuous migratory lesions of the skin caused by larvae of nematodes. A 26-year-old fisherman presented to us with complaints of an itchy eruption on his back and arms of two months’ duration. Clinical examination revealed multiple wavy serpentine tracts and fork like lesions with a raised absolute eosinophil count of 3800 cells/cmm. Biopsy was inconclusive. This case is reported to highlight the extensive involvement by larva migrans. KEY WORDS: Larva migrans, Fisherman INTRODUCTION Cutaneous larva migrans is a common tropically acquired dermatosis. It presents as an erythematous, serpiginous, pruritic, cutaneous eruption caused by percutaneous penetration and subsequent migration of larvae of various nematode parasites. CASE REPORT A 26-year-old male came with complaints of an itchy eruption on his back and arms of 2 months’ duration. He was a fisherman by occupation and gave a history of sleeping on the beach for long hours. He was treated Figure 1: Wavy serpiginous tracts with fork like lesions with antihistamines, but without any response. Cutaneous examination revealed multiple erythematous The baseline laboratory parameters were normal, with papules, plaques and wavy serpentine tracts on the back a raised absolute eosinophil count of 3800 cell/cmm. A and posterior aspect of arms (Figure 1). biopsy from the lesion showed only spongiosis with How to cite this article: Mehta VR, Shenoi SD. -

Software for Taxon-Aware Analysis of Clustered Protein Sequences
G3: Genes|Genomes|Genetics Early Online, published on September 2, 2017 as doi:10.1534/g3.117.300233 INVESTIGATIONS KinFin: Software for taxon-aware analysis of clustered protein sequences Dominik R. Laetsch∗,†,1 and Mark L. Blaxter∗ ∗Institute of Evolutionary Biology, University of Edinburgh, Edinburgh EH9 3JT UK, †The James Hutton Institute, Errol Road, Dundee DD2 5DA UK ABSTRACT The field of comparative genomics is concerned with the study of similarities and differences KEYWORDS between the information encoded in the genomes of organisms. A common approach is to define gene bioinformatics families by clustering protein sequences based on sequence similarity, and analyse protein cluster presence protein orthology and absence in different species groups as a guide to biology. Due to the high dimensionality of these data, protein family downstream analysis of protein clusters inferred from large numbers of species, or species with many genes, evolution is non-trivial, and few solutions exist for transparent, reproducible and customisable analyses. We present comparative KinFin, a streamlined software solution capable of integrating data from common file formats and delivering genomics aggregative annotation of protein clusters. KinFin delivers analyses based on systematic taxonomy of the filarial nema- species analysed, or on user-defined groupings of taxa, for example sets based on attributes such as life todes history traits, organismal phenotypes, or competing phylogenetic hypotheses. Results are reported through graphical and detailed text output files. We illustrate the utility of the KinFin pipeline by addressing questions regarding the biology of filarial nematodes, which include parasites of veterinary and medical importance. We resolve the phylogenetic relationships between the species and explore functional annotation of proteins in clusters in key lineages and between custom taxon sets, identifying gene families of interest. -

Insecurities and Dogs: an Obstacle to the Eradication of Dracunculiasis
dicine & Me S l u a r ic g e p r o y r T ISSN: 2329-9088 Tropical Medicine & Surgery Review Article Insecurities and Dogs: An Obstacle to the Eradication of Dracunculiasis Aja Kalu1*,Nwufo Amanda2 Department of Care of the Elderly, Barking, Havering and Redbridge University Hospital, NHS Trust, Romford, Essex, UK; 2 Department of Public Health, University of Chester, Chester, UK. ABSTRACT Dracunculiasis is a parasitic worm infection also known as Guinea Worm Disease (GWD). It is caused by a nematode called Dracunculiasis Medinensis. It belongs to a group of communicable disease named Neglected Tropic Disease (NTD). Dracunculiasis is caused by drinking water contaminated with the vector copepods (water fleas). Although the disease is not fatal, the sores caused by the emerging worm in the lower limb can become secondarily infected and complications such as sepsis, tetanus can ensue. Also, the sores can cause abscess and cellulitis, leaving the individual incapacitated for weeks which extends beyond the emergence of the worm. Over the last three decades, the prevalence of Guinea worm disease has reduced drastically through cost effective intervention provided by The Cater Center, WHO, UNICEF with the disease targeted for eradication. Some African countries like Nigeria, Ghana, South Africa, and Kenya being the most recent, have eliminated the disease. Guinea worm is still present in Chad, Cameroon, Mali, Ethiopia where political instability, social inequalities and infection of dogs by the worm pose an increasing threat and obstacle to the elimination of the disease. Dracunculiasis represents a disease that can be eradicated without a drug or vaccine but with a cost-effective intervention that involves community efforts. -

Identification and Characterization of the Forkhead Box
IDENTIFICATION AND CHARACTERIZATION OF THE FORKHEAD BOX FAMILY OF TRANSCRIPTIONAL REGULATORS IN PARASITIC SCHISTOSOMES by MELISSA M. VARRECCHIA Submitted in partial fulfillment of the requirements for the degree of Doctor of philosophy Department of Biology CASE WESTERN RESERVE UNIVERSITY August 2017 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the dissertation of Melissa M. Varrecchia candidate for the degree of Doctor of Philosophy Committee Chair Michael F. Benard Committee Member Emmitt R. Jolly Committee Member Christopher A. Cullis Committee Member Claudia M. Mizutani Committee Member Brian M. McDermott Date of Defense June 6, 2017 *We also certify that written approval has been obtained for any proprietary material contained therein. ii Dedication I would like to dedicate this dissertation to my Mom and Dad. Mom, thank you for your endless love, support and encouragement throughout the years. Dad, I miss you and I know that you are with me always, cheering me on in spirit. iii Table of Contents Table of Contents………………………………………………………………………...1 List of Tables……………………………………………………………………………..6 List of Figures…………………………………………………………………………....8 Acknowledgements…………………………………………………………………..…11 List of Abbreviations…………………………………………………………………...13 Abstract…………………………………………………………………………………15 Chapter 1: Introduction………………………………………………………………..17 1.1 Schistosomiasis………………………………………………………………17 1.2 Pathogenesis and treatment…………………………………………………..18 1.3 Schistosome life cycle………………………………………………………..20 1.4 Schistosome morphology -

Public Health Significance of Intestinal Parasitic Infections*
Articles in the Update series Les articles de la rubrique give a concise, authoritative, Le pointfournissent un bilan and up-to-date survey of concis et fiable de la situa- the present position in the tion actuelle dans les do- Update selectedfields, coveringmany maines consideres, couvrant different aspects of the de nombreux aspects des biomedical sciences and sciences biomedicales et de la , po n t , , public health. Most of santepublique. Laplupartde the articles are written by ces articles auront donc ete acknowledged experts on the redigeis par les specialistes subject. les plus autorises. Bulletin of the World Health Organization, 65 (5): 575-588 (1987) © World Health Organization 1987 Public health significance of intestinal parasitic infections* WHO EXPERT COMMITTEE' Intestinal parasitic infections are distributed virtually throughout the world, with high prevalence rates in many regions. Amoebiasis, ascariasis, hookworm infection and trichuriasis are among the ten most common infections in the world. Other parasitic infections such as abdominal angiostrongyliasis, intestinal capil- lariasis, and strongyloidiasis are of local or regional public health concern. The prevention and control of these infections are now more feasible than ever before owing to the discovery of safe and efficacious drugs, the improvement and sim- plification of some diagnostic procedures, and advances in parasite population biology. METHODS OF ASSESSMENT The amount of harm caused by intestinal parasitic infections to the health and welfare of individuals and communities depends on: (a) the parasite species; (b) the intensity and course of the infection; (c) the nature of the interactions between the parasite species and concurrent infections; (d) the nutritional and immunological status of the population; and (e) numerous socioeconomic factors. -

Tegegn Jaleta Phd Dissertation.Pdf
Comparative Transcriptomics and Genetic Analyses in Animal Parasitic Nematodes Dissertation der Mathematisch-Naturwissenschaftlichen Fakultät der Eberhard Karls Universität Tübingen zur Erlangung des Grades eines Doktors der Naturwissenschaften (Dr. rer. nat.) vorgelegt von Tegegn Gudeta Jaleta aus Sire, Wollega, Äthiopien Tübingen 2016 Tag der mündlichen Qualifikation: 19/12/2016 Dekan: Prof. Dr. Wolfgang Rosenstiel 1. Berichterstatter: PD Dr. Adrian Streit 2. Berichterstatter: Prof. Dr. Peter Kremsner ii Table of Contents Summary ................................................................................................................ viii Zusammenfassung .................................................................................................. ix 1. Introduction ......................................................................................................... 1 1.1 Parasitism in the phylum Nematoda ................................................................. 1 1.2 The genus Strongyloides ................................................................................. 4 1.2.1 Taxonomy ................................................................................................. 4 1.2.2 Morphology ............................................................................................... 6 1.2.3 Life cycle and reproduction ........................................................................ 6 1.2.4 Sex determination and Karyotype .............................................................. 8 1.2.5 -

Public Health Significance of Foodborne
imental er Fo p o x d E C Journal of Experimental Food f h o e l m a n i Pal et al., J Exp Food Chem 2018, 4:1 s r t u r y o J Chemistry DOI: 10.4172/2472-0542.1000135 ISSN: 2472-0542 Review Article Open Access Public Health Significance of Foodborne Helminthiasis: A Systematic Review Mahendra Pal1*, Yodit Ayele2, Angesom Hadush3, Pooja Kundu4 and Vijay J Jadhav4 1Narayan Consultancy on Veterinary Public Health, 4 Aangan, Jagnath Ganesh Dairy Road, Anand-38001, India 2Department of Animal Science, College of Agriculture and Natural Resources, Bonga University, Post Box No.334, Bonga, Ethiopia 3Department of Animal Production and Technology, College of Agriculture and Environmental Sciences, Adigrat University, P.O. Box 50, Adigrat, Ethiopia 4Department of Veterinary Public Health and Epidemiology, College of Veterinary Sciences, LUVAS, Hisar-125004, India *Corresponding author: Mahendra Pal, Narayan Consultancy on Veterinary Public Health and Microbiology, 4 Aangan, Jagnath Ganesh Dairy Road, Anand-388001, Gujarat, India, E-mail: [email protected] Received date: December 18, 2017; Accepted date: January 19, 2018; Published date: January 25, 2018 Copyright: ©2017 Pal M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Foodborne diseases, caused by biological as well as chemical agents, have an impact in both developing and developed nations. The foodborne diseases of microbial origin are acute where as those caused by chemical toxicants are resulted due to chronic exposure. -

Extracellular Onchocerca-Derived Small Rnas in Host Nodules
Edinburgh Research Explorer Extracellular Onchocerca -derived small RNAs in host nodules and blood Citation for published version: Quintana, JF, Makepeace, BL, Babayan, SA, Ivens, A, Pfarr, KM, Blaxter, M, Debrah, A, Wanji, S, Ngangyung, HF, Bah, GS, Tanya, VN, Taylor, DW, Hoerauf, A & Buck, AH 2015, 'Extracellular Onchocerca -derived small RNAs in host nodules and blood', Parasites and Vectors, vol. 8, no. 1, 58. https://doi.org/10.1186/s13071-015-0656-1 Digital Object Identifier (DOI): 10.1186/s13071-015-0656-1 Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Published In: Parasites and Vectors Publisher Rights Statement: © 2015 Quintana et al.; licensee BioMed Central. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly credited. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated. General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim.