Paralytic Shellfish Toxins in Surf Clams Mesodesma Donacium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/319999645 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Chapter · September 2017 DOI: 10.1002/9781119154051.ch10 CITATIONS READS 0 332 28 authors, including: Nelson A Lagos Ricardo Norambuena University Santo Tomás (Chile) University of Concepción 65 PUBLICATIONS 1,052 CITATIONS 13 PUBLICATIONS 252 CITATIONS SEE PROFILE SEE PROFILE Claudio Silva Marco A Lardies Pontificia Universidad Católica de Valparaíso Universidad Adolfo Ibáñez 54 PUBLICATIONS 432 CITATIONS 70 PUBLICATIONS 1,581 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Irish moss - green crab interactions View project Influence of environment on fish stock assessment View project All content following this page was uploaded by Pedro A. Quijón on 11 November 2017. The user has requested enhancement of the downloaded file. 239 10 Impacts of Climate Change on Marine Fisheries and Aquaculture in Chile Eleuterio Yáñez1, Nelson A. Lagos2,13, Ricardo Norambuena3, Claudio Silva1, Jaime Letelier4, Karl-Peter Muck5, Gustavo San Martin6, Samanta Benítez2,13, Bernardo R. Broitman7,13, Heraldo Contreras8, Cristian Duarte9,13, Stefan Gelcich10,13, Fabio A. Labra2, Marco A. Lardies11,13, Patricio H. Manríquez7, Pedro A. Quijón12, Laura Ramajo2,11, Exequiel González1, Renato Molina14, Allan Gómez1, Luis Soto15, Aldo Montecino16, María Ángela Barbieri17, Francisco Plaza18, Felipe Sánchez18, -

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Exotic / Disease Name Acronym Health Susceptible Species Vector Species Non-Exotic Listed National Status Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (C. carassius), Epizootic Declared Rainbow trout (Oncorhynchus mykiss), redfin common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Haematopoietic EHN Exotic * Disease-Free perch (Percha fluviatilis) Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench Necrosis (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), Sterlet sturgeon (Acipenser ruthenus), Starry sturgeon (Acipenser stellatus), Sturgeon (Acipenser sturio), Siberian Sturgeon (Acipenser Baerii), Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), Crucian carp (C. carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench (Tinca tinca) Herring (Cupea spp.), whitefish (Coregonus sp.), North African catfish (Clarias gariepinus), Northern pike (Esox lucius) Catfish (Ictalurus pike (Esox Lucius), haddock (Gadus aeglefinus), spp.), Black bullhead (Ameiurus melas), Channel catfish (Ictalurus punctatus), Pangas Pacific cod (G. macrocephalus), Atlantic cod (G. catfish (Pangasius pangasius), Pike perch (Sander lucioperca), Wels catfish (Silurus glanis) morhua), Pacific salmon (Onchorhynchus spp.), Viral -

Sandbridge Beach FONSI
FINDING OF NO SIGNIFICANT IMPACT Issuance of a Negotiated Agreement for Use of Outer Continental Shelf Sand from Sandbridge Shoal in the Sandbridge Beach Erosion Control and Hurricane Protection Project Virginia Beach, Virginia Pursuant to the National Environmental Policy Act (NEPA), Council on Environmental Quality regulations implementing NEPA (40 CFR 1500-1508) and Department of the Interior (DOI) regulations implementing NEPA (43 CFR 46), the Bureau of Ocean Energy Management (BOEM) prepared an environmental assessment (EA) to determine whether the issuance of a negotiated agreement for the use of Outer Continental Shelf (OCS) sand from Sandbridge Shoal Borrow Areas A and B for the Sandbridge Beach Erosion Control and Hurricane Protection Project near Virginia Beach, VA would have a significant effect on the human environment and whether an environmental impact statement (EIS) should be prepared. Several NEPA documents evaluating impacts of the project have been previously prepared by both the US Army Corps of Engineers (USACE) and BOEM. The USACE described the affected environment, evaluated potential environmental impacts (initial construction and nourishment events), and considered alternatives to the proposed action in a 2009 EA. This EA was subsequently updated and adopted by BOEM in 2012 in association with the most recent 2013 Sandbridge nourishment effort (BOEM 2012). Prior to this, BOEM (previously Minerals Management Service [MMS]) was a cooperating agency on several EAs for previous projects (MMS 1997; MMS 2001; MMS 2006). This current EA, prepared by BOEM, supplements and summarizes the aforementioned 2012 analysis. BOEM has reviewed all prior analyses, supplemented additional information as needed, and determined that the potential impacts of the current proposed action have been adequately addressed. -

The Sense of Hearing in the Pacific Oyster, Magallana Gigas
RESEARCH ARTICLE The sense of hearing in the Pacific oyster, Magallana gigas Mohcine Charifi1,2,3, Mohamedou Sow1,2, Pierre Ciret1,2, Soumaya Benomar3, Jean- Charles Massabuau1,2* 1 University of Bordeaux, EPOC, UMR 5805, Arcachon, France, 2 CNRS, EPOC, UMR 5805, Talence, France, 3 Unit of Research on Biological Rhythms, Neuroscience and Environment, Faculty of Science, Mohammed V-Agdal University, Rabat, Morocco * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract a1111111111 There is an increasing concern that anthropogenic noise could have a significant impact on the marine environment, but there is still insufficient data for most invertebrates. What do they perceive? We investigated this question in oysters Magallana gigas (Crassostrea gigas) using pure tone exposures, accelerometer fixed on the oyster shell and hydrophone in the OPEN ACCESS water column. Groups of 16 oysters were exposed to quantifiable waterborne sinusoidal Citation: Charifi M, Sow M, Ciret P, Benomar S, sounds in the range of 10 Hz to 20 kHz at various acoustic energies. The experiment was con- Massabuau J-C (2017) The sense of hearing in the ducted in running seawater using an experimental flume equipped with suspended loud- Pacific oyster, Magallana gigas. PLoS ONE 12(10): e0185353. https://doi.org/10.1371/journal. speakers. The sensitivity of the oysters was measured by recording their valve movements pone.0185353 by high-frequency noninvasive valvometry. The tests were 3 min tone exposures including a Editor: Jose A. FernaÂndez Robledo, Bigelow 70 sec fade-in period. Three endpoints were analysed: the ratio of responding individuals in Laboratory for Ocean Sciences, UNITED STATES the group, the resulting changes of valve opening amplitude and the response latency. -

Concholepas Concholepas
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 97 Band October 1985 Oktober Part 1 Deel THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPAS CONCHOLEPAS By BRIAN KENSLEY are issued in parts at irregular intervals as material becomes available word uitgegee in dele op ongereelde tye na gelang van die beskikbaarheid van stof 1,2(1-3,5-8),3(1-2,4-5,8, I.-p.i.), 5(1-3, 5, 7-9), 6(1, I.-p.i.), 7(1-4), 8, 9(1-2, 7), 10(1-3), 11(1-2,5,7, I.-p.i.), 14(1-2), 15(4-5),24(2),27,31(1-3),32(5),33,36(2),45(1) Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd., Die Rustica-pers, Edms., Bpk., Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap THE FOSSIL OCCURRENCE IN SOUTHERN AFRICA OF THE SOUTH AMERICAN INTERTIDAL MOLLUSC CONCHOLEPASCONCHOLEPAS BRIAN KENSLEY National Museum of Natural History, Smithsonian Institution, Washington, D. C. The occurrence of the thaidid gastropod genus Concholepas is recorded from presumed Late Pleistocene coastal deposits in southern South West Africa-Namibia. The material is indistinguishable from C. concholepas, a species known from the Pliocene to Recent on the west coast of South America. The living species characteristically occurs in cold-temperate waters from the intertidal to depths of 40 m. It is suggested that the southern African fossils represent a short-lived pioneer population, established by larvae drifting from South America. -

AEBR 114 Review of Factors Affecting the Abundance of Toheroa Paphies
Review of factors affecting the abundance of toheroa (Paphies ventricosa) New Zealand Aquatic Environment and Biodiversity Report No. 114 J.R. Williams, C. Sim-Smith, C. Paterson. ISSN 1179-6480 (online) ISBN 978-0-478-41468-4 (online) June 2013 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-resources/publications.aspx http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright - Ministry for Primary Industries TABLE OF CONTENTS EXECUTIVE SUMMARY ....................................................................................................... 1 1. INTRODUCTION ............................................................................................................ 2 2. METHODS ....................................................................................................................... 3 3. TIME SERIES OF ABUNDANCE .................................................................................. 3 3.1 Northland region beaches .......................................................................................... 3 3.2 Wellington region beaches ........................................................................................ 4 3.3 Southland region beaches ......................................................................................... -

Physiological Effects and Biotransformation of Paralytic
PHYSIOLOGICAL EFFECTS AND BIOTRANSFORMATION OF PARALYTIC SHELLFISH TOXINS IN NEW ZEALAND MARINE BIVALVES ______________________________________________________________ A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy in Environmental Sciences in the University of Canterbury by Andrea M. Contreras 2010 Abstract Although there are no authenticated records of human illness due to PSP in New Zealand, nationwide phytoplankton and shellfish toxicity monitoring programmes have revealed that the incidence of PSP contamination and the occurrence of the toxic Alexandrium species are more common than previously realised (Mackenzie et al., 2004). A full understanding of the mechanism of uptake, accumulation and toxin dynamics of bivalves feeding on toxic algae is fundamental for improving future regulations in the shellfish toxicity monitoring program across the country. This thesis examines the effects of toxic dinoflagellates and PSP toxins on the physiology and behaviour of bivalve molluscs. This focus arose because these aspects have not been widely studied before in New Zealand. The basic hypothesis tested was that bivalve molluscs differ in their ability to metabolise PSP toxins produced by Alexandrium tamarense and are able to transform toxins and may have special mechanisms to avoid toxin uptake. To test this hypothesis, different physiological/behavioural experiments and quantification of PSP toxins in bivalves tissues were carried out on mussels ( Perna canaliculus ), clams ( Paphies donacina and Dosinia anus ), scallops ( Pecten novaezelandiae ) and oysters ( Ostrea chilensis ) from the South Island of New Zealand. Measurements of clearance rate were used to test the sensitivity of the bivalves to PSP toxins. Other studies that involved intoxication and detoxification periods were carried out on three species of bivalves ( P. -
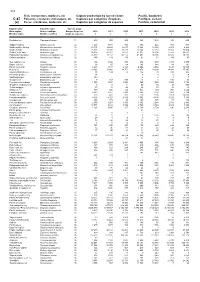
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
534 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southeast C-87 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-est (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoriental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2010 2011 2012 2013 2014 2015 2016 Nombre inglés Nombre científico Grupo de especies t t t t t t t Flatfishes nei Pleuronectiformes 31 613 302 806 323 1 804 483 693 Tadpole codling Salilota australis 32 1 400 1 091 768 374 522 703 536 Southern blue whiting Micromesistius australis 32 23 301 19 629 16 675 15 304 10 036 8 809 8 269 Southern hake Merluccius australis 32 25 361 20 909 20 288 19 346 12 393 16 150 16 804 South Pacific hake Merluccius gayi 32 90 305 82 977 72 872 92 031 96 196 77 283 98 662 Patagonian grenadier Macruronus magellanicus 32 74 330 70 137 62 175 47 602 39 138 37 475 28 108 Chilean grenadier Coelorinchus chilensis 32 156 134 136 91 54 59 47 Sea catfishes nei Ariidae 33 406 1 426 852 876 1 217 1 185 1 285 Snake eels nei Ophichthidae 33 38 65 114 142 144 49 181 Pacific cornetfish Fistularia corneta 33 6 443 4 513 4 323 4 854 2 534 7 630 19 559 Mullets nei Mugilidae 33 10 821 13 400 18 751 13 876 14 290 14 044 17 345 Snooks(=Robalos) nei Centropomus spp 33 98 104 78 136 79 310 305 Broomtail grouper Mycteroperca xenarcha 33 14 .. -

Annotated Bibliography of Fishing Impacts on Habitat - September 2003 Update
ANNOTATED BIBLIOGRAPHY OF FISHING IMPACTS ON HABITAT - SEPTEMBER 2003 UPDATE Gulf States Marine Fisheries Commission SEPTEMBER 2003 GSMFC No.: 115 Annotated Bibliography of Fishing Impacts on Habitat - September 2003 Update Edited by Jeffrey K. Rester Gulf States Marine Fisheries Commission Gulf States Marine Fisheries Commission September 2003 Introduction This is the third in a series of updates to the Gulf States Marine Fisheries Commission’s Annotated Bibliography of Fishing Impacts on Habitat originally produced in February 2000. The Commission’s Habitat Subcommittee felt that the gathering of pertinent literature should continue. The third update contains 52 new articles since the publication of the last update. The update uses the same criteria that the original bibliography and first and second updates used to compile articles. It attempts to compile a listing of papers and reports that address the many effects and impacts that fishing can have on habitat and the marine environment. The bibliography is not limited to scientific literature only. It includes technical reports, state and federal agency reports, college theses, conference and meeting proceedings, popular articles, and other forms of nonscientific literature. This was done in an attempt to gather as much information on fishing impacts as possible. Researchers will be able to decide for themselves whether they feel the included information is valuable. Fishing, both recreational and commercial, can have many varying impacts on habitat and the marine environment. Whether a fisher prop scars seagrass, drops an anchor on a coral reef, or drags a trawl across the bottom, each act can alter habitat and affect fish populations. -

Pecten Maximus) Sea-Ranching in Norway – Lessons Learned
Scallop (Pecten maximus) sea-ranching in Norway – lessons learned Ellen Sofie Grefsrud, Tore Strohmeier & Øivind Strand Background - Sea ranching in Norway • 1990-1997: Program to develop and encourage sea ranching (PUSH) • Focus on four species • Atlantic salmon (Salmo salar) • Atlantic cod (Gadus morhua) • Arctic char (Salvelinus alpinus) • European lobster (Homarus gammarus) • Only lobster showed an economic potential Grefsrud et. al, 6th ISSESR, 11-14 November, Sarasota FL, USA Background - Scallop sea ranching • 1980-90’s – a growing interest on scallop Pecten maximus cultivation in Norway • Based on suspended culture (1980’s) – labour costs high • European concensus that seeding on bottom was the most viable option • First experimental releases i Norway in mid-1990’s Photo: IMR Photo: IMR Grefsrud et. al, 6th ISSESR, 11-14 November, Sarasota FL, USA Production model Pecten maximus • Hatchery + nursery – larvae + 2-20 mm shell height • Intermediate culture – 20-55 mm • Grow-out on seabed – 55->100 mm Photo: IMR Three production steps to lower risk for investors and increase the profit in each step Time aspect – four-five years from hatchery to market sized scallops Grefsrud et. al, 6th ISSESR, 11-14 November, Sarasota FL, USA Hatchery + nursery • Established a commercial hatchery, Scalpro Photo: S. Andersen • Industry + research developed methodolgy for producing spat on a commercial scale • Larvae phase • Antibiotics and probiotica to prevent bacterial outbreaks (early phase) • Continous flow-through in larvae tank and increased volume • No use of antibiotics in commercial production • From hatching to spat in about three weeks Photo: IMR Grefsrud et. al, 6th ISSESR, 11-14 November, Sarasota FL, USA Hatchery + nursery • Transferred from hatchery to nursery in the sea at 2-4 mm • Land based race-way system – 2-20 mm Photo: IMR • Flow-through filtered sea water • Reduced predation and fouling • The commercial hatchery, Scalpro, covered both the hatchey and nursery phase Photo: IMR Grefsrud et. -

Omaha Beach: Final Archaeological Report
Omaha Beach: Final Archaeological Report Prepared for Omaha Beach Ltd 11 July 2003 Clough & Associates Ltd Simon Bickler (PhD) Heritage Consultants Matthew Campbell (PhD) www.clough.co.nz Rod Clough (PhD) [email protected] Don Prince (MA Hons) 209 Carter Rd, Oratia Mica Plowman (MA Hons) Tel. (09) 818-1316 Vanessa Tanner (MA) Mobile (0274) 850-059 Sally Burgess (MA Hons) Fax (09) 813-0112 Kim Tatton (MA Hons) Tania Mace (MA Hons) Marianne Turner (PhD) With contributions from Rod Wallace (PhD) Report Summary Archaeology The archaeology reported here is mitigation associated with earthworks on Omaha Sandspit, in two phases between July 2000 and September 2002. The spit encloses the Whangateau Harbour, which is fed by the Omaha River and the Waikokopu Creek, with alluvial and estuarine sediments forming tidal mudflats to the west, and dune sands to the east comprising old beach ridges and eroding dunes. The area was intensely used throughout the last few hundred years as a staging point for fishing and shellfish gathering, leaving behind major archaeological remains. Omaha beach is a complex and changing environment. Although the emphasis of the excavations was necessarily focussed on individual midden, the broader use of the landscape was also addressed in these investigations. The radiocarbon dates obtained from the first season were earlier than expected as it was thought that that most sites would represent the historic and proto-historic periods. However, these earlier than expected dates were repeated in the second season results and confirm that the beach had been used from about 1450 to 1750 AD. -

(Approx) Mixed Micro Shells (22G Bags) Philippines € 10,00 £8,64 $11,69 Each 22G Bag Provides Hours of Fun; Some Interesting Foraminifera Also Included
Special Price £ US$ Family Genus, species Country Quality Size Remarks w/o Photo Date added Category characteristic (€) (approx) (approx) Mixed micro shells (22g bags) Philippines € 10,00 £8,64 $11,69 Each 22g bag provides hours of fun; some interesting Foraminifera also included. 17/06/21 Mixed micro shells Ischnochitonidae Callistochiton pulchrior Panama F+++ 89mm € 1,80 £1,55 $2,10 21/12/16 Polyplacophora Ischnochitonidae Chaetopleura lurida Panama F+++ 2022mm € 3,00 £2,59 $3,51 Hairy girdles, beautifully preserved. Web 24/12/16 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 30mm+ € 4,00 £3,45 $4,68 30/04/21 Polyplacophora Ischnochitonidae Ischnochiton textilis South Africa F+++ 27.9mm € 2,80 £2,42 $3,27 30/04/21 Polyplacophora Ischnochitonidae Stenoplax limaciformis Panama F+++ 16mm+ € 6,50 £5,61 $7,60 Uncommon. 24/12/16 Polyplacophora Chitonidae Acanthopleura gemmata Philippines F+++ 25mm+ € 2,50 £2,16 $2,92 Hairy margins, beautifully preserved. 04/08/17 Polyplacophora Chitonidae Acanthopleura gemmata Australia F+++ 25mm+ € 2,60 £2,25 $3,04 02/06/18 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 41mm+ € 4,00 £3,45 $4,68 West Indian 'fuzzy' chiton. Web 24/12/16 Polyplacophora Chitonidae Acanthopleura granulata Panama F+++ 32mm+ € 3,00 £2,59 $3,51 West Indian 'fuzzy' chiton. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 44mm+ € 5,00 £4,32 $5,85 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F++ 35mm € 2,50 £2,16 $2,92 Caribbean. 24/12/16 Polyplacophora Chitonidae Chiton tuberculatus Panama F+++ 29mm+ € 3,00 £2,59 $3,51 Caribbean.