Immunohistochemistry of Great Scallop Pecten Maximus Larvae Experimentally Challenged with Pathogenic Bacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

AEBR 114 Review of Factors Affecting the Abundance of Toheroa Paphies
Review of factors affecting the abundance of toheroa (Paphies ventricosa) New Zealand Aquatic Environment and Biodiversity Report No. 114 J.R. Williams, C. Sim-Smith, C. Paterson. ISSN 1179-6480 (online) ISBN 978-0-478-41468-4 (online) June 2013 Requests for further copies should be directed to: Publications Logistics Officer Ministry for Primary Industries PO Box 2526 WELLINGTON 6140 Email: [email protected] Telephone: 0800 00 83 33 Facsimile: 04-894 0300 This publication is also available on the Ministry for Primary Industries websites at: http://www.mpi.govt.nz/news-resources/publications.aspx http://fs.fish.govt.nz go to Document library/Research reports © Crown Copyright - Ministry for Primary Industries TABLE OF CONTENTS EXECUTIVE SUMMARY ....................................................................................................... 1 1. INTRODUCTION ............................................................................................................ 2 2. METHODS ....................................................................................................................... 3 3. TIME SERIES OF ABUNDANCE .................................................................................. 3 3.1 Northland region beaches .......................................................................................... 3 3.2 Wellington region beaches ........................................................................................ 4 3.3 Southland region beaches ......................................................................................... -

Physiological Effects and Biotransformation of Paralytic
PHYSIOLOGICAL EFFECTS AND BIOTRANSFORMATION OF PARALYTIC SHELLFISH TOXINS IN NEW ZEALAND MARINE BIVALVES ______________________________________________________________ A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy in Environmental Sciences in the University of Canterbury by Andrea M. Contreras 2010 Abstract Although there are no authenticated records of human illness due to PSP in New Zealand, nationwide phytoplankton and shellfish toxicity monitoring programmes have revealed that the incidence of PSP contamination and the occurrence of the toxic Alexandrium species are more common than previously realised (Mackenzie et al., 2004). A full understanding of the mechanism of uptake, accumulation and toxin dynamics of bivalves feeding on toxic algae is fundamental for improving future regulations in the shellfish toxicity monitoring program across the country. This thesis examines the effects of toxic dinoflagellates and PSP toxins on the physiology and behaviour of bivalve molluscs. This focus arose because these aspects have not been widely studied before in New Zealand. The basic hypothesis tested was that bivalve molluscs differ in their ability to metabolise PSP toxins produced by Alexandrium tamarense and are able to transform toxins and may have special mechanisms to avoid toxin uptake. To test this hypothesis, different physiological/behavioural experiments and quantification of PSP toxins in bivalves tissues were carried out on mussels ( Perna canaliculus ), clams ( Paphies donacina and Dosinia anus ), scallops ( Pecten novaezelandiae ) and oysters ( Ostrea chilensis ) from the South Island of New Zealand. Measurements of clearance rate were used to test the sensitivity of the bivalves to PSP toxins. Other studies that involved intoxication and detoxification periods were carried out on three species of bivalves ( P. -

Omaha Beach: Final Archaeological Report
Omaha Beach: Final Archaeological Report Prepared for Omaha Beach Ltd 11 July 2003 Clough & Associates Ltd Simon Bickler (PhD) Heritage Consultants Matthew Campbell (PhD) www.clough.co.nz Rod Clough (PhD) [email protected] Don Prince (MA Hons) 209 Carter Rd, Oratia Mica Plowman (MA Hons) Tel. (09) 818-1316 Vanessa Tanner (MA) Mobile (0274) 850-059 Sally Burgess (MA Hons) Fax (09) 813-0112 Kim Tatton (MA Hons) Tania Mace (MA Hons) Marianne Turner (PhD) With contributions from Rod Wallace (PhD) Report Summary Archaeology The archaeology reported here is mitigation associated with earthworks on Omaha Sandspit, in two phases between July 2000 and September 2002. The spit encloses the Whangateau Harbour, which is fed by the Omaha River and the Waikokopu Creek, with alluvial and estuarine sediments forming tidal mudflats to the west, and dune sands to the east comprising old beach ridges and eroding dunes. The area was intensely used throughout the last few hundred years as a staging point for fishing and shellfish gathering, leaving behind major archaeological remains. Omaha beach is a complex and changing environment. Although the emphasis of the excavations was necessarily focussed on individual midden, the broader use of the landscape was also addressed in these investigations. The radiocarbon dates obtained from the first season were earlier than expected as it was thought that that most sites would represent the historic and proto-historic periods. However, these earlier than expected dates were repeated in the second season results and confirm that the beach had been used from about 1450 to 1750 AD. -

Marine Mollusca of Isotope Stages of the Last 2 Million Years in New Zealand
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/232863216 Marine Mollusca of isotope stages of the last 2 million years in New Zealand. Part 4. Gastropoda (Ptenoglossa, Neogastropoda, Heterobranchia) Article in Journal- Royal Society of New Zealand · March 2011 DOI: 10.1080/03036758.2011.548763 CITATIONS READS 19 690 1 author: Alan Beu GNS Science 167 PUBLICATIONS 3,645 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Integrating fossils and genetics of living molluscs View project Barnacle Limestones of the Southern Hemisphere View project All content following this page was uploaded by Alan Beu on 18 December 2015. The user has requested enhancement of the downloaded file. This article was downloaded by: [Beu, A. G.] On: 16 March 2011 Access details: Access Details: [subscription number 935027131] Publisher Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37- 41 Mortimer Street, London W1T 3JH, UK Journal of the Royal Society of New Zealand Publication details, including instructions for authors and subscription information: http://www.informaworld.com/smpp/title~content=t918982755 Marine Mollusca of isotope stages of the last 2 million years in New Zealand. Part 4. Gastropoda (Ptenoglossa, Neogastropoda, Heterobranchia) AG Beua a GNS Science, Lower Hutt, New Zealand Online publication date: 16 March 2011 To cite this Article Beu, AG(2011) 'Marine Mollusca of isotope stages of the last 2 million years in New Zealand. Part 4. Gastropoda (Ptenoglossa, Neogastropoda, Heterobranchia)', Journal of the Royal Society of New Zealand, 41: 1, 1 — 153 To link to this Article: DOI: 10.1080/03036758.2011.548763 URL: http://dx.doi.org/10.1080/03036758.2011.548763 PLEASE SCROLL DOWN FOR ARTICLE Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf This article may be used for research, teaching and private study purposes. -

Reproduction and Larval Development of the New Zealand Scallop, Pecten Novaezelandiae
Reproduction and larval development of the New Zealand scallop, Pecten novaezelandiae. Neil E. de Jong A thesis submitted to Auckland University of Technology in partial fulfilment of the requirements for the degree of Master of Science (MSc) 2013 School of Applied Science Table of Contents TABLE OF CONTENTS ...................................................................................... I TABLE OF FIGURES ....................................................................................... IV TABLE OF TABLES ......................................................................................... VI ATTESTATION OF AUTHORSHIP ................................................................. VII ACKNOWLEDGMENTS ................................................................................. VIII ABSTRACT ....................................................................................................... X 1 CHAPTER ONE: INTRODUCTION AND LITERATURE REVIEW .............. 1 1.1 Scallop Biology and Ecology ........................................................................................ 2 1.1.1 Diet ............................................................................................................................... 4 1.2 Fisheries and Aquaculture ............................................................................................ 5 1.2.1 Scallop Enhancement .................................................................................................. 8 1.2.2 Hatcheries ................................................................................................................. -

Shelled Molluscs
Encyclopedia of Life Support Systems (EOLSS) Archimer http://www.ifremer.fr/docelec/ ©UNESCO-EOLSS Archive Institutionnelle de l’Ifremer Shelled Molluscs Berthou P.1, Poutiers J.M.2, Goulletquer P.1, Dao J.C.1 1 : Institut Français de Recherche pour l'Exploitation de la Mer, Plouzané, France 2 : Muséum National d’Histoire Naturelle, Paris, France Abstract: Shelled molluscs are comprised of bivalves and gastropods. They are settled mainly on the continental shelf as benthic and sedentary animals due to their heavy protective shell. They can stand a wide range of environmental conditions. They are found in the whole trophic chain and are particle feeders, herbivorous, carnivorous, and predators. Exploited mollusc species are numerous. The main groups of gastropods are the whelks, conchs, abalones, tops, and turbans; and those of bivalve species are oysters, mussels, scallops, and clams. They are mainly used for food, but also for ornamental purposes, in shellcraft industries and jewelery. Consumed species are produced by fisheries and aquaculture, the latter representing 75% of the total 11.4 millions metric tons landed worldwide in 1996. Aquaculture, which mainly concerns bivalves (oysters, scallops, and mussels) relies on the simple techniques of producing juveniles, natural spat collection, and hatchery, and the fact that many species are planktivores. Keywords: bivalves, gastropods, fisheries, aquaculture, biology, fishing gears, management To cite this chapter Berthou P., Poutiers J.M., Goulletquer P., Dao J.C., SHELLED MOLLUSCS, in FISHERIES AND AQUACULTURE, from Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford ,UK, [http://www.eolss.net] 1 1. -

Executive Summary
EXECUTIVE SUMMARY The New Zealand aquaculture industry is presently dominated by the production of Greenshell™ mussels in terms of total yield, water-space utilisation and total revenue. However, there has been recent emphasis by a number of mussel farmers in the Marlborough Sounds to amend permits and consents so that other bivalve species can be farmed. In response to this, the Ministry of Fisheries commissioned the Cawthron Institute to use available information to perform a desktop investigation into the marginal differences between the environmental interactions of a range of bivalve species to underpin stocking density guidelines. A hazard assessment was used to identify the major environmental interactions between bivalves and the surrounding marine environment and this highlighted several major risk pathways, several of which were through the feeding and excretory behaviour of the bivalve crop. Marginal differences between the transfer of material by the different species were therefore investigated using a range of feeding models and environmental data from the Marlborough Sounds and Glenhaven Aquaculture Centre. The key result from analysis of the marginal differences between a range of bivalve species was that mussels generally appear to exhibit the highest clearance and excretion rates of the bivalves considered. Similarly, biodeposition intensity greater than 400 g/day/1000ind occurred most frequently in mussels (40%) followed by, scallops (33%), cupped oysters (29%), flat oysters (11%), and finally clams/cockles (6%). Overall, it appears that based on the model utilised here, the substitution of mussels, specifically Perna canaliculus, with any of the other alternate species/groups proposed would not be likely to increase either the clearance of the surrounding water, the biodeposition of suspended matter or the amount of dissolved ammonia through excretion. -

Prokaryote Infections in the New Zealand Scallops Pecten Novaezelandiae and Chlamys Delicatula
DISEASES OF AQUATIC ORGANISMS Vol. 50: 137–144, 2002 Published July 8 Dis Aquat Org Prokaryote infections in the New Zealand scallops Pecten novaezelandiae and Chlamys delicatula P. M. Hine*, B. K. Diggles National Institute of Water and Atmospheric Research, PO Box 14-901, Kilbirnie, Wellington, New Zealand ABSTRACT: Four intracellular prokaryotes are reported from the scallops Pecten novaezelandiae Reeve, 1853 and Chlamys delicatula Hutton, 1873. Elongated (1025 × 110 nm), irregular (390 × 200 nm), or toroidal (410 × 200 nm) mollicute-like organisms (M-LOs) occurred free in the cytoplasm in the digestive diverticular epithelial cells of both scallop species. Those in P. novaezelandiae bore osmiophilic blebs that sometimes connected the organisms together, and some had a rod-like protru- sion, both of which resemble the blebs and tip structures of pathogenic mycoplasmas. The M-LOs in C. delicatula had a slightly denser core than periphery. Round M-LOs, 335 × 170 nm, occurred free in the cytoplasm of agranular haemocytes in P. novaezelandiae, without apparent harm to the host cell. In P. novaezelandiae, 2 types of highly prevalent (95 to 100%) basophilic inclusions in the branchial epithelium contained Rickettsia-like organisms (R-LOs). Type 1 inclusions occurred in moderately hypertrophied, intensely basophilic cells, 8 to 10 µm in diameter, containing elongate intracellular R-LOs, 2000 × 500 nm. Type 2 inclusions were elongated and moderately basophilic in markedly hypertrophic branchial epithelial cells, 50 × 20 µm in diameter, containing -

ICES Marine Science Symposia, 215: 416—423
ICES Marine Science Symposia, 215: 416—423. 2002 The potential for ranching the scallop, Pecten maximus - past, present and future: problems and opportunities Dan Minchin Minchin, D. 2002. The potential for ranching the scallop, Pecten maximus - past, pres ent, and future: problems and opportunities. - ICES Marine Science Symposia, 215: 416-423. Ranching scallops requires a full knowledge of their biology, and this has only evolved during the last half-century. This knowledge needed to be merged with the technolog ical developments of plastics, improved power, improved navigation, and aided by legal implements. Scallop cultivation in hatcheries has greatly contributed to produc tivity of spat used as a source for several ranching programmes. Depletion of natural scallop populations has made it necessary to consider ranching as a means for creat ing a sustained resource. Few areas currently have sufficient natural settlements; when these occur, they vary in intensity from year to year. As a result, collections of wild spat cannot provide a consistent source of supply. Movements of spat may need to be controlled to maintain the diversity present in some isolated populations and to reduce disease, disease agents, and parasite transfers. Future opportunities exist for ranching scallops provided there is an improved knowledge of their interactions with other biota. Developments in biotechnology and reduced predation rates are likely to lead to significant increases in production. Flowever, the spread in the range of toxic algal events and exotic species could modify such expectations. Keywords: biology, culture, ranching, scallops. Dan Minchin: Marine Organism Investigations, 3, Marina Village, Ballina, Killaloe, Co. Clare, Ireland; tel: +35J 86 60 80 888: e-mail: [email protected]. -

Bivalve Molluscs Biology, Ecology and Culture
Bivalve Molluscs Biology, Ecology and Culture Bivalve Molluscs Biology, Ecology and Culture Elizabeth Gosling Fishing News Books An imprint of Blackwell Science © 2003 by Fishing News Books, a division of Blackwell Publishing Editorial offices: Blackwell Publishing Ltd, 9600 Garsington Road, Oxford OX4 2DQ, UK Tel: +44 (0)1865 776868 Blackwell Publishing, Inc., 350 Main Street, Malden, MA 02148-5020, USA Tel: +1 781 388 8250 Blackwell Science Asia Pty, 550 Swanston Street, Carlton,Victoria 3053, Australia Tel: +61 (0)3 8359 1011 The right of the Author to be identified as the Author of this Work has been asserted in accordance with the Copyright, Designs and Patents Act 1988. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, except as permitted by the UK Copyright, Designs and Patents Act 1988, without the prior permission of the publisher. First published 2003 Reprinted 2004 Library of Congress Cataloging-in-Publication Data Gosling, E.M. Bivalve molluscs / Elizabeth Gosling. p. cm. Includes bibliographical references. ISBN 0-85238-234-0 (alk. paper) 1. Bivalvia. I. Title. QL430.6 .G67 2002 594¢.4–dc21 2002010263 ISBN 0-85238-234-0 A catalogue record for this title is available from the British Library Set in 10.5/12pt Bembo by SNP Best-set Typesetter Ltd., Hong Kong Printed and bound in Great Britain by MPG Books Ltd, Bodmin, Cornwall The publisher’s policy is to use permanent paper from mills that operate a sustainable forestry policy, and which has been manufactured from pulp processed using acid-free and elementary chlorine-free practices. -

21St International Pectinid Workshop – Abstracts & Program
ational Pe ern ctin t t id s In W 21 o rk s h o p F/V Placopecten 1 9 t h - 2 5 th A e pr in il a 201 , M 7 ̶ Portland ABSTRACTS & PROGRAM nal Pectinid atio W rn or te ks t n h s I o p 1 2 ecten Placop F/V 1 9 e th in -2 a 5 th M A d, pri lan l 2017 ̶ Port WELCOME MESSAGE On behalf of the IPW Organizing Committee, we are delighted to welcome you to Portland, Maine, USA for the 21st International Pectinid Workshop. Some may remember the 7th IPW held here in 1989 – the organizer is a little greyer, but the enthusiasm for scallops and the out- standing venue have not waned. This Workshop follows the example of the 20th IPW that was co-hosted by two countries, Ireland and Norway. Scallops are key to the coastal economies of Canada and the United States and joint sponsorship of the IPW by the Americans and Canadi- ans was an obvious liaison. In addition to nine keynote lectures and a special ‘Industry Day’, the scientific program includes the usual assemblage of topics including fisheries, aquaculture, genetics, disease, and manage- ment. We hope that bringing some new topics and scallop enthusiasts to the meeting will en- hance your experience and perhaps bring new members to IPW extended family. There are several social events planned, including a genuine all-American baseball game and a lobster bake with traditional music. Portland is a cornucopia of good food, good music, and activ- ities – we hope you will take advantage of all it has to offer and enjoy your stay. -
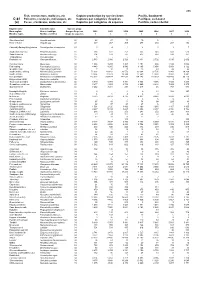
Fish, Crustaceans, Molluscs, Etc Capture Production by Species
495 Fish, crustaceans, molluscs, etc Capture production by species items Pacific, Southwest C-81 Poissons, crustacés, mollusques, etc Captures par catégories d'espèces Pacifique, sud-ouest (a) Peces, crustáceos, moluscos, etc Capturas por categorías de especies Pacífico, sudoccidental English name Scientific name Species group Nom anglais Nom scientifique Groupe d'espèces 2002 2003 2004 2005 2006 2007 2008 Nombre inglés Nombre científico Grupo de especies t t t t t t t Short-finned eel Anguilla australis 22 28 27 13 10 5 ... ... River eels nei Anguilla spp 22 337 267 209 277 210 207 152 Chinook(=Spring=King)salmon Oncorhynchus tshawytscha 23 0 4 1 2 1 1 7 Southern lemon sole Pelotretis flavilatus 31 238 322 251 335 348 608 513 Sand flounders Rhombosolea spp 31 204 193 187 437 514 530 351 Tonguefishes Cynoglossidae 31 3 - - - - - - Flatfishes nei Pleuronectiformes 31 2 580 2 986 2 729 3 431 2 702 3 015 2 602 Common mora Mora moro 32 1 308 1 234 1 403 1 154 986 1 180 1 088 Red codling Pseudophycis bachus 32 4 443 8 265 9 540 8 165 5 854 5 854 6 122 Grenadier cod Tripterophycis gilchristi 32 7 10 13 13 43 29 26 Southern blue whiting Micromesistius australis 32 72 203 43 812 26 576 30 304 32 735 23 943 29 268 Southern hake Merluccius australis 32 13 834 22 623 19 344 12 560 12 858 13 892 8 881 Blue grenadier Macruronus novaezelandiae 32 215 302 209 414 147 032 134 145 119 329 103 489 96 119 Ridge scaled rattail Macrourus carinatus 32 - - - - - 9 14 Thorntooth grenadier Lepidorhynchus denticulatus 32 5 349 5 304 6 341 3 855 4 056 3 725 3 264 Grenadiers, rattails nei Macrouridae 32 3 877 4 253 3 732 2 660 2 848 7 939 8 970 Gadiformes nei Gadiformes 32 3 252 3 281 298 1 217 46 767 886 Broadgilled hagfish Eptatretus cirrhatus 33 2 - 0 0 11 508 347 Sea catfishes nei Ariidae 33 4 6 4 4 4 ..