Amnesic Shellfish Poisoning Toxins in Bivalve Molluscs in Ireland
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Diseases Affecting Finfish
Diseases Affecting Finfish Legislation Ireland's Exotic / Disease Name Acronym Health Susceptible Species Vector Species Non-Exotic Listed National Status Disease Measures Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), crucian carp (C. carassius), Epizootic Declared Rainbow trout (Oncorhynchus mykiss), redfin common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Haematopoietic EHN Exotic * Disease-Free perch (Percha fluviatilis) Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench Necrosis (Tinca tinca) Beluga (Huso huso), Danube sturgeon (Acipenser gueldenstaedtii), Sterlet sturgeon (Acipenser ruthenus), Starry sturgeon (Acipenser stellatus), Sturgeon (Acipenser sturio), Siberian Sturgeon (Acipenser Baerii), Bighead carp (Aristichthys nobilis), goldfish (Carassius auratus), Crucian carp (C. carassius), common carp and koi carp (Cyprinus carpio), silver carp (Hypophtalmichthys molitrix), Chub (Leuciscus spp), Roach (Rutilus rutilus), Rudd (Scardinius erythrophthalmus), tench (Tinca tinca) Herring (Cupea spp.), whitefish (Coregonus sp.), North African catfish (Clarias gariepinus), Northern pike (Esox lucius) Catfish (Ictalurus pike (Esox Lucius), haddock (Gadus aeglefinus), spp.), Black bullhead (Ameiurus melas), Channel catfish (Ictalurus punctatus), Pangas Pacific cod (G. macrocephalus), Atlantic cod (G. catfish (Pangasius pangasius), Pike perch (Sander lucioperca), Wels catfish (Silurus glanis) morhua), Pacific salmon (Onchorhynchus spp.), Viral -

Pecten Maximus) Sea-Ranching in Norway – Lessons Learned
Scallop (Pecten maximus) sea-ranching in Norway – lessons learned Ellen Sofie Grefsrud, Tore Strohmeier & Øivind Strand Background - Sea ranching in Norway • 1990-1997: Program to develop and encourage sea ranching (PUSH) • Focus on four species • Atlantic salmon (Salmo salar) • Atlantic cod (Gadus morhua) • Arctic char (Salvelinus alpinus) • European lobster (Homarus gammarus) • Only lobster showed an economic potential Grefsrud et. al, 6th ISSESR, 11-14 November, Sarasota FL, USA Background - Scallop sea ranching • 1980-90’s – a growing interest on scallop Pecten maximus cultivation in Norway • Based on suspended culture (1980’s) – labour costs high • European concensus that seeding on bottom was the most viable option • First experimental releases i Norway in mid-1990’s Photo: IMR Photo: IMR Grefsrud et. al, 6th ISSESR, 11-14 November, Sarasota FL, USA Production model Pecten maximus • Hatchery + nursery – larvae + 2-20 mm shell height • Intermediate culture – 20-55 mm • Grow-out on seabed – 55->100 mm Photo: IMR Three production steps to lower risk for investors and increase the profit in each step Time aspect – four-five years from hatchery to market sized scallops Grefsrud et. al, 6th ISSESR, 11-14 November, Sarasota FL, USA Hatchery + nursery • Established a commercial hatchery, Scalpro Photo: S. Andersen • Industry + research developed methodolgy for producing spat on a commercial scale • Larvae phase • Antibiotics and probiotica to prevent bacterial outbreaks (early phase) • Continous flow-through in larvae tank and increased volume • No use of antibiotics in commercial production • From hatching to spat in about three weeks Photo: IMR Grefsrud et. al, 6th ISSESR, 11-14 November, Sarasota FL, USA Hatchery + nursery • Transferred from hatchery to nursery in the sea at 2-4 mm • Land based race-way system – 2-20 mm Photo: IMR • Flow-through filtered sea water • Reduced predation and fouling • The commercial hatchery, Scalpro, covered both the hatchey and nursery phase Photo: IMR Grefsrud et. -

Embryonic and Larval Development of Ensis Arcuatus (Jeffreys, 1865) (Bivalvia: Pharidae)
EMBRYONIC AND LARVAL DEVELOPMENT OF ENSIS ARCUATUS (JEFFREYS, 1865) (BIVALVIA: PHARIDAE) FIZ DA COSTA, SUSANA DARRIBA AND DOROTEA MARTI´NEZ-PATIN˜O Centro de Investigacio´ns Marin˜as, Consellerı´a de Pesca e Asuntos Marı´timos, Xunta de Galicia, Apdo. 94, 27700 Ribadeo, Lugo, Spain (Received 5 December 2006; accepted 19 November 2007) ABSTRACT The razor clam Ensis arcuatus (Jeffreys, 1865) is distributed from Norway to Spain and along the British coast, where it lives buried in sand in low intertidal and subtidal areas. This work is the first study to research the embryology and larval development of this species of razor clam, using light and scanning electron microscopy. A new method, consisting of changing water levels using tide simulations with brief Downloaded from https://academic.oup.com/mollus/article/74/2/103/1161011 by guest on 23 September 2021 dry periods, was developed to induce spawning in this species. The blastula was the first motile stage and in the gastrula stage the vitelline coat was lost. The shell field appeared in the late gastrula. The trocho- phore developed by about 19 h post-fertilization (hpf) (198C). At 30 hpf the D-shaped larva showed a developed digestive system consisting of a mouth, a foregut, a digestive gland followed by an intestine and an anus. Larvae spontaneously settled after 20 days at a length of 378 mm. INTRODUCTION following families: Mytilidae (Redfearn, Chanley & Chanley, 1986; Fuller & Lutz, 1989; Bellolio, Toledo & Dupre´, 1996; Ensis arcuatus (Jeffreys, 1865) is the most abundant species of Hanyu et al., 2001), Ostreidae (Le Pennec & Coatanea, 1985; Pharidae in Spain. -

Panopea Abrupta ) Ecology and Aquaculture Production
COMPREHENSIVE LITERATURE REVIEW AND SYNOPSIS OF ISSUES RELATING TO GEODUCK ( PANOPEA ABRUPTA ) ECOLOGY AND AQUACULTURE PRODUCTION Prepared for Washington State Department of Natural Resources by Kristine Feldman, Brent Vadopalas, David Armstrong, Carolyn Friedman, Ray Hilborn, Kerry Naish, Jose Orensanz, and Juan Valero (School of Aquatic and Fishery Sciences, University of Washington), Jennifer Ruesink (Department of Biology, University of Washington), Andrew Suhrbier, Aimee Christy, and Dan Cheney (Pacific Shellfish Institute), and Jonathan P. Davis (Baywater Inc.) February 6, 2004 TABLE OF CONTENTS LIST OF FIGURES ........................................................................................................... iv LIST OF TABLES...............................................................................................................v 1. EXECUTIVE SUMMARY ....................................................................................... 1 1.1 General life history ..................................................................................... 1 1.2 Predator-prey interactions........................................................................... 2 1.3 Community and ecosystem effects of geoducks......................................... 2 1.4 Spatial structure of geoduck populations.................................................... 3 1.5 Genetic-based differences at the population level ...................................... 3 1.6 Commercial geoduck hatchery practices ................................................... -

Pecten Maximus
Assessing the association between scallops, Pecten Maximus, and the benthic ecosystem within the Isle of Man marine reserves A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science (MSc) in Marine Environmental Protection. Chyanna Allison Bsc Marine Biology with International Experience (2015, Bangor University) Supervisor: Michel J. Kaiser, Isobel Bloor and Jack Emmerson School of Ocean Sciences, College of Natural Sciences Bangor University, Gwynedd, LL57 2UW, UK September 2016 DECLARATION This work has not previously been accepted in substance for any degree and is not being concurrently submitted in candidature for any degree. Signed: Chyanna Allison Date: 16/09/16 STATEMENT 1: This thesis is the result of my own investigations, except where otherwise stated. Where correction services have been used, the extent and nature of the correction is clearly marked in a footnote(s). Other sources are acknowledged by footnotes giving explicit references. A bibliography is appended. Signed: Chyanna Allison Date: 16/09/16 STATEMENT 2: I hereby give consent for my thesis, if accepted, to be available for photocopying and for inter- library loan, and for the title and summary to be made available to outside organisations. Signed: Chyanna Allison Date: 16/09/16 NB: Candidates on whose behalf a bar on access has been approved by the University should use the following version of Statement 2: I hereby give consent for my thesis, if accepted, to be available for photocopying and for inter- library loans after expiry of a bar on access approved by the University. Signed: Chyanna Allison Date: 16/09/16 i | P a g e Abstract The increased threat to the world’s marine environment has forced the use of management strategies that encourage resource sustainability like spatial closure and stock replenishment. -

Reproduction and Larval Development of the New Zealand Scallop, Pecten Novaezelandiae
Reproduction and larval development of the New Zealand scallop, Pecten novaezelandiae. Neil E. de Jong A thesis submitted to Auckland University of Technology in partial fulfilment of the requirements for the degree of Master of Science (MSc) 2013 School of Applied Science Table of Contents TABLE OF CONTENTS ...................................................................................... I TABLE OF FIGURES ....................................................................................... IV TABLE OF TABLES ......................................................................................... VI ATTESTATION OF AUTHORSHIP ................................................................. VII ACKNOWLEDGMENTS ................................................................................. VIII ABSTRACT ....................................................................................................... X 1 CHAPTER ONE: INTRODUCTION AND LITERATURE REVIEW .............. 1 1.1 Scallop Biology and Ecology ........................................................................................ 2 1.1.1 Diet ............................................................................................................................... 4 1.2 Fisheries and Aquaculture ............................................................................................ 5 1.2.1 Scallop Enhancement .................................................................................................. 8 1.2.2 Hatcheries ................................................................................................................. -

Abundance, Age/Size Structure and Fecundity of Pecten Maximus and Aequipecten Opercularis Inside and Outside a Temperate No Take Zone
Summer placement report Exam number: Y0011466 Gimme Shell-ter: Abundance, age/size structure and fecundity of Pecten maximus and Aequipecten opercularis inside and outside a temperate no take zone ABSTRACT: The effectiveness of strict spatial protection as a shellfish fisheries management tool was examined through the collection and measurement of two sample sets of two commercially harvested pectinid species: Pecten maximus and Aequipecten opercularis. One sample set was collected from locations inside an unfished “no take zone” in the Firth of Clyde, Scotland and the other from fished locations surrounding this reserve; sampled P. maximus individuals inside the reserve were 1.4 times older and 1.3 times larger than those sampled outside, with A. opercularis displaying the same differences in age and size. Exploitable biomass was 1.6 times higher among sampled P. maximus inside the reserve and 2 times higher among A. opercularis individuals, with similar scale differences observed for both species’ reproductive biomass. In spite of these clear differences in age, size and biomass, neither species was found to be more abundant inside the reserve nor was consistent evidence found that reserve populations were exhibiting faster growth characteristics than those outside. The lack of strong comparative density patterns is attributed to minimal scallop fishery pressure in the “fished” sample locations and possible population stability engendered by the older, larger protected individuals. A broader, “network” approach of reserves is recommended for the area’s shellfish fisheries, in line with similar methodologies implemented in the Irish Sea. 1 Summer placement report Exam number: Y0011466 1. Introduction Commercially exploited mollusc species make up 7.2% of global capture fishery production, 12.7% of which comprises individuals of species in the Pectinidae family, or scallops (Food and Agriculture Organization of the United Nations [FAO], 2011). -

Effects of Hydrodynamic Factors on Pecten Maximus Larval Development Marine Holbach, Rene Robert, Philippe Miner, Christian Mingant, Pierre Boudry, Rejean Tremblay
Effects of hydrodynamic factors on Pecten maximus larval development Marine Holbach, Rene Robert, Philippe Miner, Christian Mingant, Pierre Boudry, Rejean Tremblay To cite this version: Marine Holbach, Rene Robert, Philippe Miner, Christian Mingant, Pierre Boudry, et al.. Effects of hydrodynamic factors on Pecten maximus larval development. Aquaculture Research, Wiley, 2017, 48 (11), pp.5463-5471. 10.1111/are.13361. hal-02577608 HAL Id: hal-02577608 https://hal.archives-ouvertes.fr/hal-02577608 Submitted on 14 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 1 Aquaculture Research Achimer November 2017, Volume 48, Issue 11, Pages 5463-5471 http://dx.doi.org/10.1111/are.13361 http://archimer.ifremer.fr http://archimer.ifremer.fr/doc/00384/49508/ © 2017 John Wiley & Sons Ltd Effects of hydrodynamic factors on Pecten maximus larval development Holbach Marine 1, 2, * , Robert Rene 3, Miner Philippe 2, Mingant Christian 2, Boudry Pierre 2, Tremblay Réjean 1 1 Institut des Sciences de la Mer; Université du Québec à Rimouski; Rimouski QC ,Canada 2 Laboratoire des Sciences de l'Environnement Marin (LEMAR UMR 6539 UBO/CNRS/IRD/Ifremer); Centre Bretagne; Ifremer; Plouzané ,France 3 Unité Littoral; Centre Bretagne; Ifremer; Plouzané, France * Corresponding author : Marine Holbach, email address : [email protected] Abstract : Hatchery production of great scallop, Pecten maximus, remains unpredictable, notably due to poor larval survival. -

1 Strategic Environmental Assessment
Strategic Environmental Assessment - SEA5 Technical Report for Department of Trade & Industry NORTHERN NORTH SEA SHELLFISH AND FISHERIES Prepared by: Colin J Chapman Bloomfield Milltimber Aberdeenshire AB13 0EQ 1 NORTHERN NORTH SEA SHELLFISH AND FISHERIES Prepared by: Colin J Chapman Contents Executive Summary ................................................................................................ 4 1. Introduction ............................................................................................... 10 2. Shellfish resources..................................................................................... 10 2.1 Fishery data ........................................................................................ 10 2.2 Crustacean species 2.2.1 Norway lobster, Nephrops norvegicus (L.).............................. 11 2.2.2 European lobster, Homarus gammarus (L.)............................. 16 2.2.3 Edible crab, Cancer pagurus (L.)............................................. 20 2.2.4 Velvet swimming crab, Necora puber (L.) .............................. 23 2.2.5 Shore crab, Carcinus maenus (L.)............................................ 25 2.2.6 Pink shrimp, Pandalus borealis Kroyer................................... 26 2.2.7 Other species ............................................................................ 28 2.3 Bivalve molluscs 2.3.1 Scallop, Pecten maximus (L.)................................................... 29 2.3.2 Queen scallop, Aequipecten opercularis (L.)........................... 32 2.3.3 Cockle, -

The Abundance, Movement and Site Fidelity of the Adult Whelk, Buccinum Undatum, in the Territorial Waters of the Isle of Man
The abundance, movement and site fidelity of the adult whelk, Buccinum undatum, in the territorial waters of the Isle of Man A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science (MSc) in Marine Environmental Protection Bangor University 2015 Matthew Robinson BSc Marine Biology (2010, University of Liverpool) School of Ocean Sciences, Bangor University, Menai Bridge, Anglesey, LL59 5EY, UK www.bangor.ac.uk [email protected] Submitted in September, 2015 DECLARATION This work has not previously been accepted in substance for any degree and is not being currently submitted for any degree. Candidate: Date: 18/09/2015 This dissertation is being submitted in partial fulfillment of the requirement of the MSc in Marine Environmental Protection. Candidate: Date: 18/09/2015 The dissertation is the result of my own independent work / investigation, except where otherwise stated. Candidate: Date: 18/09/2015 I hereby give consent for my dissertation, if accepted, to be made available for photocopying and for inter-library loan, and the title and summary to be made available to outside organisations. Candidate: Date: 18/09/2015 Acknowledgements I would like to thank Professor Michel Kaiser, Dr Isobel Bloor and Sam Dignan for their support and guidance during the project. I am immensely grateful to Jon Jo Skillen, skipper of ‘Boy Shayne’, and Jordan Corkill for their efforts and enthusiasm for making the project a success. Thank you also to Lucy May for her help during the tag making and tagging phase of the project, and for her support throughout. The abundance, movement and site fidelity of the adult whelk, Buccinum undatum, in the territorial waters of the Isle of Man Abstract The maximum and daily movements of the common whelk (Buccinum undatum) were investigated in Ramsey Bay, northeast Isle of Man from June to July 2015. -

And OYSTERS (Crassostrea Gigas) Nicole DEVAUCHELLE§
SPERM MOVEMENT AND FECUNDANCE IN SCALLOPS(Pecten maximus) and OYSTERS (Crassostrea gigas) Nicole DEVAUCHELLE§. Jacky COSSON@,Laurent ORSINI§,Catherine FAURE§ and Jean-Pierre GIRARD£ §=IFREMER, Direction desRessources Vivantes, BP 70, Pointe du Diable, F29280 Plouzanné FRANCE. @=URA671 du CNRS, University of Paris 6, Marine Station, 06230, Villefranche/mer, FRANCE. £= Univ. Nice Sophia-Antipolis, BP 71, 06108 NICE, FRANCE Introduction : In oyster (Oy=successive hermaphrodite) and scallop (Sc=simultaneous hermaphrodite) both with external fertilization, spermatozoa (=spz) have a quite long active period of several hours in sea water (SW). In Oy, most of the spz show flagellar erratic bending devoid of regular waves, leading to poorly efficient translationnal efficiency. In order to improve the fertilization performances, we have succeeded to design conditions where almost 100% of the Oy spz exhibit efficient forward motility, thanks to incubation with polyvinylpyrollidone (PVP) in sea water, major changes in flagellar waves shape are observed, mostly regular waves along the axoneme from base to tip . Such spz induced to high performance of swim were used successfully for insemination of mature oocytes. Material & Methods : Biol, materials: spz (45-50pm long) and oocytes (70pm diam.) of oysters (Crassostrea gigas) & scallops (Pecten maximum were collected either: 1 )after a thermal shock of spawners conditionned in large SW tanks(similar to natural spawning) or 2)by gonad scarification, or 3)by collection following serotonin treatment with a seringe applied to the genital pore (dry sp=1011 to 1012 sp/ml as assessed by spectrophotometry at 209 nm). Fertilization tests: at 100 spz/ovocyte and contact time of gametes = 30 min for Oy and 60-120 min for Sc. -
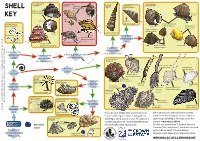
Shell Whelk Dog Whelk Turret It Could Be a Periwinkle Shell (Nucella Lapillus) Shell Spire Shell Thick Top Shell (Osilinus Lineatus) Dark Stripes Key on Body
It could be a type of It could be a type of It could be a It could be a type of topshell whelk Dog whelk turret It could be a periwinkle Shell (Nucella lapillus) shell spire shell Thick top shell (Osilinus lineatus) Dark stripes Key on body Egg Underside capsules Actual size It could be a type of (Hydrobia sp) Common periwinkle spiral worm White ‘Colar’ (Littorina littorea) Flat periwinkle (Littorinasp) Yes Roughly ‘ribbed’ shell. Very high up shore ‘Tooth inside (Turitella communis) opening (Spirorbis sp) Does it have 6 Common whelk No (Buccinum undatum) Yes or more whorls Brown, speckled Netted dog whelk body (twists)? Painted topshell (Nassarius reticulatus) (Calliostoma zizyphinum) No Rough periwinkle Flattened spire Yes Is it long, thin (Littorina saxatilis) Yes Yes and cone shaped Is it permanently No like a unicorn’s horn? attached to Is there a groove or teeth No Is there mother No a surface? in the shell opening? of pearl inside It could be a type of the shell opening? bivalve Yes Yes Common otter-shell (Lutraria lutraria) Bean-like tellin No Is the shell in (Fabulina fabula) Is it 2 parts? spiraled? Common cockle (Cerastoderma edule) It could be a Flat, rounded No sand No Great scallop mason It could be a Is the shell a (Pecten maximus) shell Razor shell worm keel worm Wedge-shaped Is the case dome or (Ensis sp) No Pacific oyster shell made from Yes cone shape? (Crassostrea gigas) Shell can be Peppery furrow shell very large (Scrobicularia plana) sand grains? Elongated and and doesn’t (Lanice conchilega) deep-bodied fully close with large ‘frills’ No (Pomatoceros sp) Yes It could be a type of sea urchin It could be a type of An acorn Native oyster Empty barnacle barnacle Does it have that may be found in estuaries and shores in the UK.