Evaluation of the Environmental Risk from the Co-Deposition of Waste
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Incised and Impressed Pottery During the Neolithic Period in Western Macedonia
Incised and impressed pottery during the Neolithic period in Western Macedonia Magdalini Tsigka SCHOOL OF HUMANITIES A thesis submitted for the degree of Master of Arts (MA) in the Classical Archaeology and the Ancient History of Macedonia December 2018 Thessaloniki – Greece 2 Student Name: Magdalini Tsigka SID: 2204150030 Supervisor: Prof. S. M. Valamoti I hereby declare that the work submitted is mine and that where I have made use of another’s work, I have attributed the source(s) according to the Regulations set in the Student’s Handbook. December 2018 Thessaloniki - Greece 3 Preface This study is the completion of the postgraduate course of MA in the Classical Archaeology and the Ancient History of Macedonia at the International University of Thessaloniki. In order for this thesis to be completed, the contribution of some people was important. First of all, I would like to thank Prof. S. M. Valamoti who accepted to supervise my thesis and encouraged me in all its stages. I would also like to thank Dr. A. Dimoula who helped me throughout all the steps for its completion, from finding the subject up to the end of my work. She was always present to direct me and to solve any questions or concerns about the subject. Then I want to thank L. Gkelou, archaeologist of the Ephorate of Florina, for entrusting me material from the excavation of Anargyroi VIIc and made this study possible despite all the adversities. Also, I would like to thank the Director of the Ephorate of Florina, Dr C. Ziota, for the discussion and the information she gave me during my study of the material. -

EUROPEAN SOCIAL CHARTER the GOVERNMENT of GREECE • Follow up to Collective Complaints • Complementary Information on Article
28/08/2015 RAP/Cha/GRC/25(2015) EUROPEAN SOCIAL CHARTER 25th National Report on the implementation of the European Social Charter submitted by THE GOVERNMENT OF GREECE Follow up to Collective Complaints Complementary information on Articles 11§2 and 13§4 (Conclusions 2013) __________ Report registered by the Secretariat on 28 August 2015 CYCLE XX-4 (2015) 25th Greek Report on the European Social Charter Follow-up to the decisions of the European Committee of Social Rights relating to Collective Complaints (2000 – 2012) Ministry of Labour, Social Security & Social Solidarity May 2015 25th Greek Report on the European Social Charter TABLE OF CONTENTS 1. Collective Complaint 8/2000 “Quaker Council for European Affairs v. Greece” .......... 4 2. Collective Complaints (a) 15/2003, “European Roma Rights Centre [ERRC] v. Greece” & (b) 49/2008, “International Centre for the Legal Protection for Human Rights – [INTERIGHTS] v. Greece” ........................................................................................................ 8 3. Collective Complaint 17/2003 “World Organisation against Torture [OMCT] v. Greece” ................................................................................................................................. 12 4. Collective Complaint 30/2005 “Marangopoulos Foundation for Human Rights v. Greece” ................................................................................................................................. 19 5. Collective Complaint “General Federation of Employees of the National Electric -

UCLA Electronic Theses and Dissertations
UCLA UCLA Electronic Theses and Dissertations Title Cremation, Society, and Landscape in the North Aegean, 6000-700 BCE Permalink https://escholarship.org/uc/item/8588693d Author Kontonicolas, MaryAnn Emilia Publication Date 2018 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Los Angeles Cremation, Society, and Landscape in the North Aegean, 6000 – 700 BCE A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Archaeology by MaryAnn Kontonicolas 2018 © Copyright by MaryAnn Kontonicolas 2018 ABSTRACT OF THE DISSERTATION Cremation, Society, and Landscape in the North Aegean, 6000 – 700 BCE by MaryAnn Kontonicolas Doctor of Philosophy in Archaeology University of California, Los Angeles, 2018 Professor John K. Papadopoulos, Chair This research project examines the appearance and proliferation of some of the earliest cremation burials in Europe in the context of the prehistoric north Aegean. Using archaeological and osteological evidence from the region between the Pindos mountains and Evros river in northern Greece, this study examines the formation of death rituals, the role of landscape in the emergence of cemeteries, and expressions of social identities against the backdrop of diachronic change and synchronic variation. I draw on a rich and diverse record of mortuary practices to examine the co-existence of cremation and inhumation rites from the beginnings of farming in the Neolithic period -

The Licit and the Illicit in Archaeological and Heritage Discourses
CHALLENGING THE DICHOTOMY EDIT ED BY LES FIELD CRISTÓBAL GNeccO JOE WATKINS CHALLENGING THE DICHOTOMY • The Licit and the Illicit in Archaeological and Heritage Discourses TUCSON The University of Arizona Press www.uapress.arizona.edu © 2016 by The Arizona Board of Regents Open-access edition published 2020 ISBN-13: 978-0-8165-3130-1 (cloth) ISBN-13: 978-0-8165-4169-0 (open-access e-book) The text of this book is licensed under the Creative Commons Atrribution- NonCommercial-NoDerivsatives 4.0 (CC BY-NC-ND 4.0), which means that the text may be used for non-commercial purposes, provided credit is given to the author. For details go to http://creativecommons.org/licenses/by-nc-nd/4.0/. Cover designed by Leigh McDonald Publication of this book is made possible in part by the Wenner-Gren Foundation. Library of Congress Cataloging-in-Publication Data Names: Field, Les W., editor. | Gnecco, Cristóbal, editor. | Watkins, Joe, 1951– editor. Title: Challenging the dichotomy : the licit and the illicit in archaeological and heritage discourses / edited by Les Field, Cristóbal Gnecco, and Joe Watkins. Description: Tucson : The University of Arizona Press, 2016. | Includes bibliographical references and index. Identifiers: LCCN 2016007488 | ISBN 9780816531301 (cloth : alk. paper) Subjects: LCSH: Archaeology. | Archaeology and state. | Cultural property—Protection. Classification: LCC CC65 .C47 2016 | DDC 930.1—dc23 LC record available at https:// lccn.loc.gov/2016007488 An electronic version of this book is freely available, thanks to the support of libraries working with Knowledge Unlatched. KU is a collaborative initiative designed to make high quality books Open Access for the public good. -

Aiani—Historical and Geographical Context
CHAPTER 5 AIANI—HISTORICAL AND GEOGRAPHICAL CONTEXT G. Karamitrou-Mentessidi Aiani—Elimiotis—Upper Macedonia According to the foundation myth preserved by Stephanos of Byzantium, “Aiani, a Macedonian city, was built by Aianos, a son of Elymos the Tyr- rhenian king, who migrated to Macedonia, ‘Aianaios’ was the ethnikon.” A reference to the city of Aiani was found by the French academic L. Heuzey in 1861 in two inscriptions in churches in the village of Kalliani (whose name may derive from “kali Aiani,” meaning “fair Aiani,”: in 1926 it was renamed Aiani). They led to various theories about the site of the settle- ment and its urban organization. The problem was not solved until offfijicial excavations began. Even if an urban settlement by the name of Elimeia did also exist, Aiani became the capital of Elimiotis at a very early date. The district of Elimeia or Elimiotis, bordering Orestis and Eordaea to the north, occupied the southern part of Upper Macedonia on both sides of the middle reach of the River Haliacmon, although its exact territorial extent is difffijicult to determine. During the Hellenistic era it appears to have included the region of Tymphaea to the west and, perhaps at an earlier date, the northern part of the Perrhaebian Tripolitis (Pythium, Azorus, and Doliche). Territorial disputes between Elimeia and its neigh- bours occurred, as can be seen in a Latin inscription found at Doliche, in which the emperor Trajan (101 ad) defijined the boundary between the Elimiotae and the inhabitants of Doliche by reviving an earlier ruling by King Amyntas III (393–369 bc) on the same issue. -

Hellenic Geosphaera Exploration (I.G.M.E.) Special Issue March 2008
Institute of Geology and Mineral Hellenic Geosphaera Exploration (I.G.M.E.) Special Issue March 2008 CONTENTS Newsletter of the Institute of Geology and Mineral Exploration (I.G.M.E.) Introductory address from the General 1 L.E.P.L. Supervised by the Ministry Director of the I.G.M.E. of Development (L. 272/76) Director General Prof. A. Georgakopoulos Hellenic industrial minerals and rocks: 2 current research performed by the I.G.M.E., Editorial Board: by N. Kaklamanis EurGeol Alecos Demetriades The modern filler laboratory of I.G.M.E. 6 Dr. Irene Zananiri Alexandra Zervakou Potential industrial applications of vein- 7 Athanasios Makris quartz resources in Northern Hellas, Dr. Michalis Patronis by N. Arvanitidis Dr. Athanasios Hatzikirkou Mineralogical - Petrographical laboratories 14 Editing of this issue: Applied mineralogy for the efficient 15 EurGeol Alecos Demetriades exploitation of wasted magnesite Run-Of- Fotini Chalkiopoulou Mine fines, by F. Chalkiopoulou, M. Grossou-Valta, S. Karantassi I.G.M.E. Central Offices: “LITHOS”: The accredited ornamental stone 20 1, Spirou Loui, str. quality control laboratory of the I.G.M.E. Olympic Village, Entrance C 136 77 Acharnae Hellenic marble through the ages: an 21 overview of the marble producing areas and Tel. +30 210 2413000 the stone sector of today, Fax +30 210 2413015 by K. Laskaridis http://www.igme.gr/ The contribution of petrography to the 27 Edition distributed free of charge. evaluation of carbonate aggregates for Articles represent the views of the author(s). concrete production, by M. Dimitroula Quotation / reproduction is permitted only with proper citation of the source. -

IV. NORDGRIECHENLAND THESSALIEN Geologie
755 IV. NORDGRIECHENLAND THESSALIEN Lit.: Zusammenfassend: Chourmouziadis 1982. Andreou S. – Fotiadis – Kotsakis 1996, 539–560. Andreou S. – Fotiadis – Kotsakis, 2001, 261–282. Leekley – Efstratiou 1980, 129–160. Zur chronologischen Terminologie und der Definition von „Frühthessalisch“ (FTh) und „Mittelthessalisch“ (MTh) siehe im ersten Band das Kapitel zur Chro- nologie. Geologie Lit.: Demitrack 1994. Besios – Krahtopoulou 2001. Kambouroglou 1994. Papageorgiou – Steiros – Chourmouziadis 1994. Steiros – Papageorgiou 1992. Steiros – Papageorgiou 1994. van Andel – Zangger – Demitrack 1990. van Andel – Zangger 1990. Zangger 1991b. Die geologischen Untersuchungen in Thessalien betrafen eine Anzahl von Phänomenen, welche die Lage von Siedlungen in prähistorischer Zeit in ein ande- res Licht rücken, aber auch Neuerkenntnisse für ihre wirtschaftliche Grundlage liefern. Inhalt von Studien sind Anstieg und Fall des Meeresspiegels, tektonische Veränderungen, die Alluviation der Ebenen, der Einschnitt und Verlauf der Flüs- se, Größenveränderungen der Seen und die Evidenz von Vulkanausbrüchen in Südthessalien. Von Bedeutung waren auch anthropogene Effekte wie die Entwal- dung der Landschaft, wogegen klimatologische Veränderungen eine geringere Rolle gespielt haben. Letztendlich zeigen diese Studien auch, daß Fundplätze aufgrund der Alluviation verschüttet sein können.1 Am pagasäischen Golf fand eine ständige Verlandung durch das Schwemmaterial der Flüsse statt. Studien zeigen, daß Petromagula in der Frühbronzezeit am Meer lag.2 Die Alluviation der 1 Papageorgiou – Steiros – Chourmouziadis 1994. Steiros – Papageorgiou 1992. Steiros – Papageorgiou 1994. 2 Kambouroglou 1994. Papageorgiou – Steiros – Chourmouziadis 1994. Steiros – Papa- georgiou 1992. Steiros – Papageorgiou 1994. Zangger 1991b. Thessalien 755 Ebene von Larissa ist Inhalt mehrerer Studien, die zeigen, daß die frühbronzezeit- lichen Siedlungen auf dem Girtoni-Alluvium liegen.3 Besiedlung Lit.: Feuer 1983. Halstead 1984. Gallis 1992. -

Winter 2008.Pmd
VOLUME 30 • NUMBER 119 • WINTER 2008 ΟΡΓΑΝΟΝ ΤΩΝ ΑΠΑΝΤΑΧΟΥ ΙΚΑΡΙΩΝ OFFICIAL MAGAZINE OF THE PAN-ICARIAN BROTHERHOOD OF AMERICA AND THE PAN-ICARIAN FOUNDATION OF AMERICA Ikapia Magazine Page 1 IKARIA MAGAZINE IS A PUBLICATION OF THE PAN-ICARIAN BROTHERHOOD OF AMERICA, “ICAROS” PAN-ICARIAN BROTHERHOOD OF AMERICA NATIONAL HEADQUARTERS 721 8th Street, Oakmont , PA 15139 Telephone: (w) 412-828-9666, ext 33 (h) 412-828-4947 Email: [email protected] 2007-2008 SUPREME OFFICERS OF THE PAN-ICARIAN BROTHERHOOD SUPREME PRESIDENT MIKE AIVALIOTIS 721 8th Street, Oakmont, PA 15139 Telephone: (w) 412-828-9666, ext 33 (h) 412-828-4947 Email: [email protected] or [email protected] SUPREME VICE PRESIDENT, SONJA STEFANADIS 460 Palm Island SE, Clearwater, FL 33767 Telephone: 727-447-2715 Email: [email protected] SUPREME SECRETARY, NIKOLAOS J. PASAMIHALIS 1756 Gross Avenue, Pennsauken , NJ 08110 Telephone: (w) 215-925-6565, (h) 856-662-7426 Email: [email protected] or [email protected] SUPREME TREASURER /DATABASE MGR., GEORGE KOKLANARIS 22236 Harlan, Grosse Ile , MI 48138 Telephone: (w) 734-283-1277, (h) 734-676-9307 Email: [email protected] COUNSELOR, ATHINA SIRINGAS 1441 St. Antoine, 12th Floor, Detroit, MI 48226 Telephone: (w) 313 224-6642 Email: [email protected] 2007-2008 FOUNDATION OFFICERS Pan-Icarian Foundation P.O. Box 79037 Pittsburgh, PA 15216-0037 Chairman Socrates Koutsoutis, 1757 Elton Road, Suite 210 Silver Spring, MD 20903 301-439-7788 [email protected] Vice Chairman C.D. “Gus” Yiakas, 1248 Via Coronel, Palos Verdes Estates, CA 90274 310-378-3984 [email protected] Director Chris Aivaliotis, 222 Park Square Lane, Pittsburgh, PA 15238 (w) 412-828-9666 (ext 30) [email protected] Director PNP John A. -

Winter 2006 Magazine
VOLUME 28 • NUMBER 113 • WINTER 2006 ΟΡΓΑΝΟΝ ΤΩΝ ΑΠΑΝΤΑΧΟΥ ΙΚΑΡΙΩΝ OFFICIAL MAGAZINE OF THE PAN-ICARIAN BROTHERHOOD OF AMERICA AND THE PAN-ICARIAN FOUNDATION OF AMERICA A CONVENTION OF GRAND PROPORTIONS THE GRAND BANQUET 102nd ANNUAL PAN-ICARIAN CONVENTION ~ MYRTLE BEACH, SC HOSTED BY CHAPTER THERMA #10 SEPTEMBER 4, 2005 Ikapia Magazine Page 1 IKARIA MAGAZINE IS A PUBLICATION OF THE PAN-ICARIAN BROTHERHOOD OF AMERICA, “ICAROS” PAN-ICARIAN BROTHERHOOD OF AMERICA NATIONAL HEADQUARTERS 18320 Heatherlea Drive Livonia, MI 48152 Telephone: (w) 313-965-3040, (h) 734-462-0034 Email: [email protected] 2005-2006 SUPREME OFFICERS OF THE PAN-ICARIAN BROTHERHOOD SUPREME PRESIDENT NICHOLAS J. TSALIS 18320 Heatherlea Drive Livonia, MI 48152 Telephone: (w) 313-965-3040, (h) 734-462-0034 Email: [email protected] SUPREME VICE PRESIDENT, MIKE AIVALIOTIS 721 8th Street Oakmont, PA 15139 Telephone: (w) 412-828-9666 (ext 33), (h) 412-828-4947 Email: [email protected] SUPREME SECRETARY, SONJA STEFANADIS 460 Palm Island SE Clearwater, FL 33767 Telephone: 727-447-2715 Email: [email protected] SUPREME TREASURER, NIKOLAOS J. PASAMIHALIS 1756 Gross Avenue Pennsauken, NJ 08110 Telephone: (w) 215-925-6565, (h) 856-662-7426 Email: [email protected] COUNSELOR, E. TERRY PLATIS 321 S. Sangamon, #808 Chicago, IL 60607 Telephone: (w) 312-861-2044, (h) 312-563-0036 Email: [email protected] or [email protected] 2004-2005 FOUNDATION OFFICERS • Pan-Icarian Foundation P.O. Box 79037 Pittsburgh, PA 15216-0037 Chairman Anthony Kayafas, 215-44 29th Ave. Bayside, NY 11360-2651 (w) 718-458-5545, (h) 718-224-3454 [email protected] Vice Chairman Socrates Koutsoutis, 1757 Elton Road, Suite 210 Silver Spring, MD 20903 301-439-7788 [email protected] Director Chris Aivaliotis, 701 Brunot Street Verona, PA 15147 (w) 412-828-9666 (ext 30), (h) [email protected] Director C.D. -
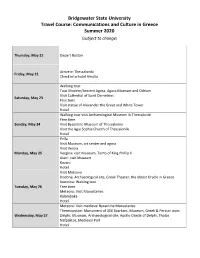
Faculty Led Program Schedule
Bridgewater State University Travel Course: Communications and Culture in Greece Summer 2020 (subject to change) Thursday, May 21 Depart Boston Arrive in Thessaloniki Friday, May 22 Check into hotel Amalia Walking tour Tour Modern/Ancient Agora, Agora Museum and Odeum Visit Cathedral of Saint Demetrios Saturday, May 23 Free time Visit statue of Alexander the Great and White Tower Hotel Walking tour visit Archaeological Museum in Thessaloniki Free time Sunday, May 24 Visit Byzantine Museum of Thessaloniki Visit the Agia Sophia Church of Thessaloniki Hotel Pella Visit Museum, art center and agora Visit Veroia Monday, May 25 Vergina: visit Museum, Tomb of King Phillip II Aiani: visit Museum Kozani Hotel Visit Metsovo Dodona, Archaeological site, Greek Theater, the oldest Oracle in Greece Ioannina: Walking tour Tuesday, May 26 Free time Meteora: Visit Monasteries Kalambaka Hotel Meteora: Visit medieval Byzantine Monasteries Thermopylaw: Monument of 300 Spartans, Museum, Greek & Persian wars Wednesday, May 27 Delphi: Museum, Archaeological site, Apollo Oracle of Delphi, Tholos Nafpaktos, Medieval Port Hotel Ancient Olympia. Visit Museums, Archaeological site – Ancient Olympic Stadium Lagadia Thursday, May 28 Nemea. Visit archaeological site and museum. Visit Nemean wineries Argos Hotel Epidauros. Visit Museum, Asklipieion archaeological site, Ancient Greek Theater of Epidauris Mycenae. Visit Museum, Lions Gate with Ancient Acropolis, Mycenaean Friday, May 29 Tombs of Klytaimnystra Isthmus of Corinth Athens, check into hotel Free time Walking -

Religious Tourism Religious Tourism 2 of Greece the Otherface Make Themostof It! Greeks Overthecenturies; Itisatripthroughtime
Religious tourism www.visitgreece.gr RELIGIOUS TOURISM IS NOT A NOVELTY. TRAVELLING FOR RELIGIOUS PURPOSES HAS BEEN THE PRINCIpaL REASON FOR TRAVEL, SINCE THE ISM_2 R DAWN OF HUMAN HISTORY. In Greece, religious tourism stems from pilgrimage-related Greeks have always expressed their religiousness, their deep The other face activities, well-rooted in past ages. Since deep antiquity, pil- faith and devotion to God for two thousand years, keeping to Or- grimage has been a strong incentive for travelling and people thodox principles. journeyed all over Greece to visit religious sites. Moreover, the Foreign and Greek visitors alike stand in astonishment before the cultural aspect of religion is closely related to tourism, making it thousands of Byzantine churches, the innumerable chapels, the a special kind of tourist activity based on different cultural back- monasteries and their dependencies, the convents, the holy pil- RELIGIOUS TOU of Greece grounds and traditions. grimage sites and the countless other awe-inspiring religious places. Orthodox practice has been associated in many areas with con- structions and monuments of worship of various religions, and this brings out the special historical and cultural value of Greece. Whether on a trip for religious purposes or just for sight-seeing, visitors can’t help admiring the wonderful spots on the islands or the mainland, in places of worship such as monasteries and churches. Thousands of tourists each year visit the Byzantine and post- Byzantine works of art, the mosaics, murals and icons as well as other religious sites, and this shows their interest in traditions and the abiding connection between Art and Religion. -

Bibliography of Aegean Prehistory and Related Areas
Volume 31 Number 4 Pages 3853-3868 April 2004 ISSN 0028-2812 [email protected] Bibliography of Aegean Prehistory and Related Areas Published monthly, September to May, by the Department of Classics, University of Cincinnati P.O. Box 0226, Cincinnati, Ohio 45221-0226, U.S.A. Editor : Carol Hershenson Assistant Editors: K. Mark Armstrong, Sarah Dieterle, Kalliopi Efkleidou, Julie Hruby, Shannon LaFayette COMMUNICATIONS From the Editors The editors of Nestor bid farewell to assistant editor Michael Ludwig, and wish him well in his future endeavors. We gratefully thank former assistant editor K. Mark Armstrong who voluntarily helped put together this issue along with new assistant editor Julie Hruby whom we welcome. Announcements Dr. Ioannis Georganas is proposing to launch a new academic journal entitled Studies in Greek Early Iron Age Archaeology and would be interested in hearing from individuals interested in contributing as co-editors, members of the editorial advisory board, or article authors. This journal is intended to provide a forum in which to discuss issues related to the archaeology of the Greek Early Iron Age (ca. 1100-700 BCE), a period crucial for Greek history, although underestimated compared to the Bronze Age and the Classical period, including theoretical and methodological perspectives as well as interpretation of material culture. More information is available from Dr. Ioannis Georganas at [email protected]. The semi-popular quarterly Near Eastern Archaeology (NEA), published by the American Schools of Oriental Research, is soliciting brief articles for its “Arti-Facts” section. “Arti-Facts” is an ideal venue to highlight recent expeditions and discoveries, museum exhibitions, conference proceedings, and dissertation research.