Acknowledgments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Papilio Glaucus, P. Marcellus, P. Philenor, Pieris Rapae, Colias Philo Dice, Antho Caris Genutia, Anaea Andria, Euptychia Gemma
102 REMINGTON: 1952 Central Season Vol.7, nos.3·4 Papilio glaucus, P. marcellus, P. philenor, Pieris rapae, Colias philo dice, Antho caris genutia, Anaea andria, Euptychia gemma. One exception to the general scarcity was the large number of Erynnis brizo and E. juvenalis which were seen clustered around damp spots in a dry branch on April 9. MERRITT counted 67 Erynnis and 2 Papilio glaucus around one such spOt and 45 Erynnis around another. Only one specimen of Incisalia henrici was seen this spring. MERRITT was pleased to find Incisalia niphon still present in a small tract of pine although the area was swept by a ground fire in 1951. Vanessa cardui appeared sparingly from June 12 on, the first since 1947. In the late summer the season appeared normal. Eurema lisa, Nathalis iole, Lycaena thoe, and Hylephila phyleus were common. Junonia coenia was more abundant around Louisville than he has ever seen it. A rarity taken in Louisville this fall was Atlides halesus, the first seen since 1948. The latest seasonal record made by Merritt was a specimen of Colias eury theme flying south very fast on December 7. EDWARD WELLING sent a record of finding Lagoa crispata on June 27 at Covington. Contributors: F. R. ARNHOLD; E. G. BAILEY; RALPH BEEBE; S. M. COX; H. V. DALY; 1. W . GRIEWISCH; J. B. HAYES; R. W. HODGES; VONTA P. HYNES; R. LEUSCHNER; J. R. MERRITT; J. H. NEWMAN; M. C. NIEL SEN; 1. S. PHILLIPS; P. S. REMINGTON; WM. SIEKER; EDWARD VOSS; W. H . WAGNER, JR.; E. C. -

A Reconnaissance of Population Genetic Variation in Arctic and Subarctic Sulfur Butterflies (Colias Spp.; Lepidoptera, Pieridae)
1614 A reconnaissance of population genetic variation in arctic and subarctic sulfur butterflies (Colias spp.; Lepidoptera, Pieridae) Christopher W. Wheat, Ward B. Watt, and Christian L. Boutwell Abstract: Genotype–phenotype–environment interactions in temperate-zone species of Colias Fabricius, 1807 have been well studied in evolutionary terms. Arctic and alpine habitats present a different range of ecological, especially thermal, conditions under which such work could be extended across species and higher clades. To this end, we survey variation in three genes that code for phosphoglucose isomerase (PGI), phosphoglucomutase (PGM), and glucose-6-phosphate dehydrogenase (G6PD) in seven arctic and alpine Colias taxa (one only for G6PD). These genes are highly polymor- phic in all taxa studied. Patterns of variation for the PGI gene in these northern taxa suggest that the balancing selec- tion seen at this gene in temperate-zone taxa may extend throughout northern North America. Comparative study of these taxa may thus give insight into the mechanisms driving genetic differentiation among subspecies, species, and broader clades, supporting the study of both micro- and macro-evolutionary questions. Résumé : L’étude des interactions génotype–phénotype–environnement chez les papillons Colias Fabricius, 1807 de la région tempérée s’est faite dans une perspective évolutive. Les habitats arctiques et alpins offrent une gamme différente de conditions écologiques et, en particulier, thermiques dans lesquelles un tel travail peut s’étendre au niveau des espè- ces et des clades supérieurs. Dans ce but, nous avons étudié la variation de trois gènes — ceux de la phosphoglucose isomérase (PGI), de la phosphoglucomutase (PGM) et de la glucose-6-phosphate déshydrogénase (G6PD) — chez sept taxons de Colias arctiques et alpins (un seul taxon pour G6PD). -

PROGRAM ZÁCHRANY Žltáčika Zanoväťového (Colias Myrmidone Esper, 1781) Na Obdobie 2021 – 2025 (Aktualizácia Programu Záchrany Z Roku 2016)
Štátna ochrana prírody Slovenskej republiky Banská Bystrica PROGRAM ZÁCHRANY žltáčika zanoväťového (Colias myrmidone Esper, 1781) na obdobie 2021 – 2025 (aktualizácia programu záchrany z roku 2016) Spracovali: Mgr. Soňa Štefaniková, PhD., Ing. Ivana Havranová, PhD., Spolupráca: Ľubomír Víťaz Banská Bystrica, marec 2021 Ministerstvo životného prostredia Slovenskej republiky schválilo materiál 15. apríla 2021 Obsah 1. SÚČASNÝ STAV ............................................................................................................................ 3 1.1. Rozšírenie a stav populácie ...................................................................................................... 3 1.1.1. Zaradenie druhu v medzinárodnom a národnom sozologickom zozname ..................... 3 1.1.2. Zhodnotenie rozšírenia druhu v medzinárodnom meradle ............................................. 3 1.1.3. Zhodnotenie rozšírenia druhu na území Slovenskej republiky ...................................... 4 1.1.4. Zoznam nepotvrdených, neoverených a zaniknutých lokalít a príčiny ich zániku ......... 4 1.1.5. Zoznam potvrdených lokalít s analýzou stavu populácie druhu na lokalite ................... 6 1.2. Biologické a ekologické nároky ............................................................................................... 7 1.3. Faktory ohrozenia ..................................................................................................................... 9 1.4. Doterajšie zabezpečenie ochrany........................................................................................... -

Butterflies (Lepidoptera: Hesperioidea, Papilionoidea) of the Kampinos National Park and Its Buffer Zone
Fr a g m e n t a Fa u n ist ic a 51 (2): 107-118, 2008 PL ISSN 0015-9301 O M u seu m a n d I n s t i t u t e o f Z o o l o g y PAS Butterflies (Lepidoptera: Hesperioidea, Papilionoidea) of the Kampinos National Park and its buffer zone Izabela DZIEKAŃSKA* and M arcin SlELEZNlEW** * Department o f Applied Entomology, Warsaw University of Life Sciences, Nowoursynowska 159, 02-776, Warszawa, Poland; e-mail: e-mail: [email protected] **Department o f Invertebrate Zoology, Institute o f Biology, University o f Białystok, Świerkowa 2OB, 15-950 Białystok, Poland; e-mail: [email protected] Abstract: Kampinos National Park is the second largest protected area in Poland and therefore a potentially important stronghold for biodiversity in the Mazovia region. However it has been abandoned as an area of lepidopterological studies for a long time. A total number of 80 butterfly species were recorded during inventory studies (2005-2008), which proved the occurrence of 80 species (81.6% of species recorded in the Mazovia voivodeship and about half of Polish fauna), including 7 from the European Red Data Book and 15 from the national red list (8 protected by law). Several xerothermophilous species have probably become extinct in the last few decadesColias ( myrmidone, Pseudophilotes vicrama, Melitaea aurelia, Hipparchia statilinus, H. alcyone), or are endangered in the KNP and in the region (e.g. Maculinea arion, Melitaea didyma), due to afforestation and spontaneous succession. Higrophilous butterflies have generally suffered less from recent changes in land use, but action to stop the deterioration of their habitats is urgently needed. -

Papilio (New Series) #24 2016 Issn 2372-9449
PAPILIO (NEW SERIES) #24 2016 ISSN 2372-9449 MEAD’S BUTTERFLIES IN COLORADO, 1871 by James A. Scott, Ph.D. in entomology, University of California Berkeley, 1972 (e-mail: [email protected]) Table of Contents Introduction………………………………………………………..……….……………….p. 1 Locations of Localities Mentioned Below…………………………………..……..……….p. 7 Summary of Butterflies Collected at Mead’s Major Localities………………….…..……..p. 8 Mead’s Butterflies, Sorted by Butterfly Species…………………………………………..p. 11 Diary of Mead’s Travels and Butterflies Collected……………………………….……….p. 43 Identity of Mead’s Field Names for Butterflies he Collected……………………….…….p. 64 Discussion and Conclusions………………………………………………….……………p. 66 Acknowledgments………………………………………………………….……………...p. 67 Literature Cited……………………………………………………………….………...….p. 67 Table 1………………………………………………………………………….………..….p. 6 Table 2……………………………………………………………………………………..p. 37 Introduction Theodore L. Mead (1852-1936) visited central Colorado from June to September 1871 to collect butterflies. Considerable effort has been spent trying to determine the identities of the butterflies he collected for his future father-in-law William Henry Edwards, and where he collected them. Brown (1956) tried to deduce his itinerary based on the specimens and the few letters etc. available to him then. Brown (1964-1987) designated lectotypes and neotypes for the names of the butterflies that William Henry Edwards described, including 24 based on Mead’s specimens. Brown & Brown (1996) published many later-discovered letters written by Mead describing his travels and collections. Calhoun (2013) purchased Mead’s journal and published Mead’s brief journal descriptions of his collecting efforts and his travels by stage and horseback and walking, and Calhoun commented on some of the butterflies he collected (especially lectotypes). Calhoun (2015a) published an abbreviated summary of Mead’s travels using those improved locations from the journal etc., and detailed the type localities of some of the butterflies named from Mead specimens. -

MOTHS and BUTTERFLIES LEPIDOPTERA DISTRIBUTION DATA SOURCES (LEPIDOPTERA) * Detailed Distributional Information Has Been J.D
MOTHS AND BUTTERFLIES LEPIDOPTERA DISTRIBUTION DATA SOURCES (LEPIDOPTERA) * Detailed distributional information has been J.D. Lafontaine published for only a few groups of Lepidoptera in western Biological Resources Program, Agriculture and Agri-food Canada. Scott (1986) gives good distribution maps for Canada butterflies in North America but these are generalized shade Central Experimental Farm Ottawa, Ontario K1A 0C6 maps that give no detail within the Montane Cordillera Ecozone. A series of memoirs on the Inchworms (family and Geometridae) of Canada by McGuffin (1967, 1972, 1977, 1981, 1987) and Bolte (1990) cover about 3/4 of the Canadian J.T. Troubridge fauna and include dot maps for most species. A long term project on the “Forest Lepidoptera of Canada” resulted in a Pacific Agri-Food Research Centre (Agassiz) four volume series on Lepidoptera that feed on trees in Agriculture and Agri-Food Canada Canada and these also give dot maps for most species Box 1000, Agassiz, B.C. V0M 1A0 (McGugan, 1958; Prentice, 1962, 1963, 1965). Dot maps for three groups of Cutworm Moths (Family Noctuidae): the subfamily Plusiinae (Lafontaine and Poole, 1991), the subfamilies Cuculliinae and Psaphidinae (Poole, 1995), and ABSTRACT the tribe Noctuini (subfamily Noctuinae) (Lafontaine, 1998) have also been published. Most fascicles in The Moths of The Montane Cordillera Ecozone of British Columbia America North of Mexico series (e.g. Ferguson, 1971-72, and southwestern Alberta supports a diverse fauna with over 1978; Franclemont, 1973; Hodges, 1971, 1986; Lafontaine, 2,000 species of butterflies and moths (Order Lepidoptera) 1987; Munroe, 1972-74, 1976; Neunzig, 1986, 1990, 1997) recorded to date. -

CBD First National Report
FIRST NATIONAL REPORT OF THE REPUBLIC OF SERBIA TO THE UNITED NATIONS CONVENTION ON BIOLOGICAL DIVERSITY July 2010 ACRONYMS AND ABBREVIATIONS .................................................................................... 3 1. EXECUTIVE SUMMARY ........................................................................................... 4 2. INTRODUCTION ....................................................................................................... 5 2.1 Geographic Profile .......................................................................................... 5 2.2 Climate Profile ...................................................................................................... 5 2.3 Population Profile ................................................................................................. 7 2.4 Economic Profile .................................................................................................. 7 3 THE BIODIVERSITY OF SERBIA .............................................................................. 8 3.1 Overview......................................................................................................... 8 3.2 Ecosystem and Habitat Diversity .................................................................... 8 3.3 Species Diversity ............................................................................................ 9 3.4 Genetic Diversity ............................................................................................. 9 3.5 Protected Areas .............................................................................................10 -

Study of Fluorescent Pigments in Lepidoptera by Means of Paper Partition Chromatography!
1968 Journal of the Lepidopterists' Society 27 STUDY OF FLUORESCENT PIGMENTS IN LEPIDOPTERA BY MEANS OF PAPER PARTITION CHROMATOGRAPHY! GEORGE W. RAWSON2 10405 Amherst Ave., Silver Spring, Maryland Subsequent to the extensive study of organic pigments in Lepidoptera by Ford (1941-1955), very little has been done to advance our knowl edge of pigments in butterflies. There have been, however, some special ized investigations by a group of biochemists and geneticists interested in the chemical structure of pteridine compounds (Hadorn, 1962). Having been interested in butterflies during my youth and as an avocation for over half a century, I have devoted a number of years after retirement in an attempt to continue research on organic pigments in butterflies by employing the comparatively new yet popular technique of paper partition chromatography. Based on studies of fluorescent pig ments of many species, genera and families of butterflies on chromato grams, including the distribution of pigments in various parts of the body, I hope this paper will be of interest to fellow lepidopterists. Sufficient evidence has been obtained to show that the pigments in Lepidoptera and other orders of insects, particularly the fluorescent pteridines, are correlated to morphological taxonomy and that this prin ciple can be a valuable auxiliary aid in systematics. HISTORICAL REVIEW Apparently, the first person to study thc chemistry of pigments in butterflies was Hopkins (1891, 1895 a, b, c). He discovered two water soluble pigments, leucopterin and xanthopterin, in the wings of white and yellow pierid butterflies, respectively. The chemical structure of these compounds, however, was not known until they were re-examined by Wieland and SchopP They are regarded as purine compounds and are called pterins or pteridines, the name being derived from the Greek work for wing "ptcron." Thirty years after Hopkins' papers, Cockayne (1924) made a study of reactions of butterflies' wings when examined 1 Contribution No. -

Yukon Butterflies a Guide to Yukon Butterflies
Wildlife Viewing Yukon butterflies A guide to Yukon butterflies Where to find them Currently, about 91 species of butterflies, representing five families, are known from Yukon, but scientists expect to discover more. Finding butterflies in Yukon is easy. Just look in any natural, open area on a warm, sunny day. Two excellent butterfly viewing spots are Keno Hill and the Blackstone Uplands. Pick up Yukon’s Wildlife Viewing Guide to find these and other wildlife viewing hotspots. Visitors follow an old mining road Viewing tips to explore the alpine on top of Keno Hill. This booklet will help you view and identify some of the more common butterflies, and a few distinctive but less common species. Additional species are mentioned but not illustrated. In some cases, © Government of Yukon 2019 you will need a detailed book, such as , ISBN 978-1-55362-862-2 The Butterflies of Canada to identify the exact species that you have seen. All photos by Crispin Guppy except as follows: In the Alpine (p.ii) Some Yukon butterflies, by Ryan Agar; Cerisy’s Sphynx moth (p.2) by Sara Nielsen; Anicia such as the large swallowtails, Checkerspot (p.2) by Bruce Bennett; swallowtails (p.3) by Bruce are bright to advertise their Bennett; Freija Fritillary (p.12) by Sonja Stange; Gallium Sphinx presence to mates. Others are caterpillar (p.19) by William Kleeden (www.yukonexplorer.com); coloured in dull earth tones Butterfly hike at Keno (p.21) by Peter Long; Alpine Interpretive that allow them to hide from bird Centre (p.22) by Bruce Bennett. -
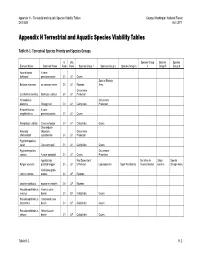
Appendix H Terrestrial and Aquatic Species Viability Tables
Appendix H – Terrestrial and Aquatic Species Viability Tables George Washington National Forest Draft EIS April 2011 Appendix H Terrestrial and Aquatic Species Viability Tables Table H‐1. Terrestrial Species Priority and Species Groups. G Unit Species Group Species Species Element Name Common Name Rank Rank Species Group 1 Species Group 2 Species Group 3 4 Group 5 Group 6 Apochthonius A cave holsingeri pseudoscorpion G1 U1 Caves Special Biologic Boltonia montana no common name G1 U1 Riparian Area Occurrence Corallorhiza bentleyi Bentley's coalroot G1 U1 Protection Helicodiscus Occurrence diadema Shaggy coil G1 U1 Calciphiles Protection Kleptochthonius A cave anophthalmus pseudoscorpion G1 U1 Caves Nampabius turbator Cave centipede G1 U1 Calciphiles Caves Shenandoah Nannaria Mountain Occurrence shenandoah xystodesmid G1 U1 Protection Pygmarrhopalites sacer Cave springtail G1 U1 Calciphiles Caves Pygmarrhopalites Occurrence caedus A cave springtail G1 U1 Caves Protection Appalachian Fire Dependent/ Sensitive to Shale Special Pyrgus wyandot grizzled skipper G1 U1 Enhanced Lepidopterans Open Woodlands Over-Collection barrens Biologic Area Kankakee globe- Iliamna remota mallow G1 UP Riparian Leuctra monticola montane needlefly G1 UP Riparian Pseudanophthalmus Avernus cave avernus beetle G1 UP Calciphiles Caves Pseudanophthalmus Crossroads cave intersectus beetle G1 UP Calciphiles Caves Pseudanophthalmus Nelson's cave nelsoni beetle G1 UP Calciphiles Caves Table H‐1 H‐1 Appendix H – Terrestrial and Aquatic Species Viability Tables George Washington -

Lepidoptera: Pieridae
Journal of Entomology and Zoology Studies 2017; 5(2): 600-604 E-ISSN: 2320-7078 P-ISSN: 2349-6800 JEZS 2017; 5(2): 600-604 Life cycle of nilgiri clouded yellow Colias © 2017 JEZS Received: 16-01-2017 nilagiriensis C. & R. Felder [Lepidoptera: Pieridae] Accepted: 17-02-2017 Udhagamandalam, the Nilgiris, Tamil Nadu Jeevith S The Wynter Blyth Association, #7D Plains View Garden, Tiger Jeevith S and S Manoj Hill, Coonoor, The Nilgiris – 643 101, Tamil Nadu, India Abstract S Manoj The life cycle of Nilgiri Clouded Yellow butterfly Colias nilagiriensis C. & R. Felder, larval The Wynter Blyth Association, performance and life cycle on its new host plant Trifolium repens L. family Fabaceae was recorded and #7D Plains View Garden, Tiger described for the first time. The study was carried out during post monsoon in the year 2016 at Hill, Coonoor, The Nilgiris – 643 Udhagamandalam, the Nilgiris district, Tamil Nadu. Fresh eggs of Nilgiri Clouded Yellow were collected 101, Tamil Nadu, India from its host plant. Colias nilagiriensis completed its life cycle from egg to adult in 55-62 days. The larvae stage lasted 24-26 days followed pupa stage which lasted for 20-25 days. Keywords: Life cycle, Colias nilagiriensis, Trifolium repens, Udhagamandalam, Nilgiris 1. Introduction The genus Colias Fabricius, 1807 is usually difficult to reach a taxonomic decision on [1] phenotypic difference . Colias nilagiriensis C. & R. Felder Nilgiri Clouded Yellow belongs to the whites and yellows family Pieridae and sub-family Coliadinae. It is a small butterfly endemic to Southern Western Ghats of India at an elevation 1,900 m above [2]. -

Maine State Legislature
MAINE STATE LEGISLATURE The following document is provided by the LAW AND LEGISLATIVE DIGITAL LIBRARY at the Maine State Law and Legislative Reference Library http://legislature.maine.gov/lawlib Reproduced from scanned originals with text recognition applied (searchable text may contain some errors and/or omissions) l 'j l Public Documents of Maine: BEING THE ANNUAL REPORTS OF THE VARIOUS PuOlic Officers an~ Institutions FOR THE YEAR 188 4. VOLUME II. AUGUSTA: SPRAGUE & SON, PRINTERS TO THE STATE. 188 4. Dormitory and Boarding House. White Hall. Laborat')ry. PR:NC:PAL Bu:Ln:~~Gs OF TH:·: S·rA'1'2 CoLLEGE o>· AGR:CULTURE AN:> '!'HE M:,:cHAn:c ARTS, Oiw:w. ANNUAL REPORTS OF THE Trustees, President, Farm Superintendent aml Treasurer OF THE STATE COLLEGE OF AGRICULTURE AND THE MECHANIC ARTS, Orono, Me., 1883. Published agreeably to a Resolve approved February 25, 187 J. , AUGUSTA: SPRAGUE & SON, PRINTERS TO THE STATE. 18 8 4. ' TRUSTEES' REPORT. To his Excellency, t!te Governor, ancl Executive Council of the State of 1~J,aine: The trustees of the State college respectfully submit their sixteenth annual report, . together with the reports of the president nnd members of the Board of Instruction, and of the trensurer of the college. These several reports will show the present condition of the institution. In response to a recommendation of the trnstees in their report of last year, the Legislature appropriated $2,800 to build and equip shops to afford facilities for the instruction of students in several branches of shop work. The plan outlined in the report referred to has been carried out.