Structure of Hard and Soft Carapace Exoskeleton Biomaterial Through SEM-EDXRS at Various Stages of Development Scylla Paramamosain Mud Crab
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

A Systematic and Experimental Analysis of Their Genes, Genomes, Mrnas and Proteins; and Perspective to Next Generation Sequencing
Crustaceana 92 (10) 1169-1205 CRUSTACEAN VITELLOGENIN: A SYSTEMATIC AND EXPERIMENTAL ANALYSIS OF THEIR GENES, GENOMES, MRNAS AND PROTEINS; AND PERSPECTIVE TO NEXT GENERATION SEQUENCING BY STEPHANIE JIMENEZ-GUTIERREZ1), CRISTIAN E. CADENA-CABALLERO2), CARLOS BARRIOS-HERNANDEZ3), RAUL PEREZ-GONZALEZ1), FRANCISCO MARTINEZ-PEREZ2,3) and LAURA R. JIMENEZ-GUTIERREZ1,5) 1) Sea Science Faculty, Sinaloa Autonomous University, Mazatlan, Sinaloa, 82000, Mexico 2) Coelomate Genomic Laboratory, Microbiology and Genetics Group, Industrial University of Santander, Bucaramanga, 680007, Colombia 3) Advanced Computing and a Large Scale Group, Industrial University of Santander, Bucaramanga, 680007, Colombia 4) Catedra-CONACYT, National Council for Science and Technology, CDMX, 03940, Mexico ABSTRACT Crustacean vitellogenesis is a process that involves Vitellin, produced via endoproteolysis of its precursor, which is designated as Vitellogenin (Vtg). The Vtg gene, mRNA and protein regulation involve several environmental factors and physiological processes, including gonadal maturation and moult stages, among others. Once the Vtg gene, mRNAs and protein are obtained, it is possible to establish the relationship between the elements that participate in their regulation, which could either be species-specific, or tissue-specific. This work is a systematic analysis that compares the similarities and differences of Vtg genes, mRNA and Vtg between the crustacean species reported in databases with respect to that obtained from the transcriptome of Callinectes arcuatus, C. toxotes, Penaeus stylirostris and P. vannamei obtained with MiSeq sequencing technology from Illumina. Those analyses confirm that the Vtg obtained from selected species will serve to understand the process of vitellogenesis in crustaceans that is important for fisheries and aquaculture. RESUMEN La vitelogénesis de los crustáceos es un proceso que involucra la vitelina, producida a través de la endoproteólisis de su precursor llamado Vitelogenina (Vtg). -

Effect of Feed on the Growth and Survival of Long Eyed Swimming
Soundarapandian et al., 2:3 http://dx.doi.org/10.4172/scientificreports681 Open Access Open Access Scientific Reports Scientific Reports Research Article OpenOpen Access Access Effect of Feed on the Growth and Survival of Long Eyed Swimming Crab Podophthalmus vigil Fabricius (Crustacea: Decapoda) Soundarapandian P1*, Ravichandran S2 and Varadharajan D1 1Faculty of Marine Sciences, Centre of Advanced Study in Marine Biology, Annamalai University, Tamil Nadu, India 2Department of Zoology, Government Arts College, Kumbakonam, Tamil Nadu, India Abstract Food is considered to be the most potent factor affecting growth. Attempts to develop diets for culture of crabs have resulted in a variety of feeds. The absence of suitable feed either pellet or live food which can promote growth and survival is considered to be the most important lacuna in cultivation of crabs. So searching of economically viable feed to optimize the growth and survival in crabs are very much essential. In the present study the weight gain was higher in the crabs offered with Acetes sp. (86 g) followed by clam meat fed animals (47 g). The crabs fed with minimum amount of Acetes sp. (152 g) and maximum of clam meat (182 g). The FCR value was better in Acetes sp. (1.8) fed animal rather than clam meat fed animals (3.8). The survival rate was higher in Acetes sp. fed animals (92%) and lowest survival (72%) was observed in animals fed with clam meat. The survival was reasonable for both the feeds. But higher survival rate was reported in the animals fed with Acetes sp. than that of clam meat. -

Redalyc.Crecimiento Del Camarón Excavador Parastacus Pugnax
Latin American Journal of Aquatic Research E-ISSN: 0718-560X [email protected] Pontificia Universidad Católica de Valparaíso Chile Ibarra, Mauricio; Arana, Patricio M. Crecimiento del camarón excavador Parastacus pugnax (Poeppig, 1835) determinado mediante técnica de marcaje Latin American Journal of Aquatic Research, vol. 39, núm. 2, julio, 2011, pp. 378-384 Pontificia Universidad Católica de Valparaíso Valparaiso, Chile Disponible en: http://www.redalyc.org/articulo.oa?id=175019398018 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Lat. Am. J. Aquat. Res., 39(2): 378-384, 2011 Lat. Am. J. Aquat. Res. 378 DOI: 10.3856/vol39-issue2-fulltext-18 Short Communication Crecimiento del camarón excavador Parastacus pugnax (Poeppig, 1835) determinado mediante técnica de marcaje Mauricio Ibarra1 & Patricio M. Arana1 1Escuela de Ciencias del Mar, Pontificia Universidad Católica de Valparaíso P.O. Box 1020, Valparaíso, Chile RESUMEN. Para determinar el crecimiento en el camarón excavador (Parastacus pugnax) en la zona centro sur de Chile se utilizó un marbete tipo cinturón. Los parámetros longitud cefalotorácica asintótica (Lc∞) y la velocidad de incremento en longitud y peso (K), se establecieron mediante el método de Gulland & Holt (1959). El parámetro t0 se determinó mediante la ecuación inversa del modelo de von Bertalanffy, estableciéndose que las curvas de crecimiento en longitud y peso fueron definidas por los parámetros K = 0,35 -1 mm año , t0 = -0,38 años, Lc∞ = 55,9 mm y W∞ = 83,8 g, valores similares a los de Samastacus spinifrons, especie chilena de alto potencial de cultivo, y de otros parastácidos sudamericanos, tales como P. -

Full Text in Pdf Format
Vol. 25: 83–96, 2016 AQUATIC BIOLOGY Published August 24 doi: 10.3354/ab00663 Aquat Biol OPENPEN ACCESSCCESS Thermal adaptations of embryos of six terrestrial hermit crab species Katsuyuki Hamasaki1,*, Takahiro Matsuda1, Ken Takano1, Mio Sugizaki1, Yu Murakami1, Shigeki Dan2, Shuichi Kitada1 1Graduate School of Marine Science and Technology, Tokyo University of Marine Science and Technology, Minato, Konan, Tokyo 108-8477, Japan 2Research Centre for Marine Invertebrate, National Research Institute of Fisheries and Environment of Inland Sea, Fisheries Research Agency, Momoshima, Onomichi, Hiroshima 722-0061, Japan ABSTRACT: We evaluated the thermal adaptations of embryos of 6 terrestrial hermit crab species in the family Coenobitidae (genera Birgus and Coenobita): B. latro, C. brevimanus, C. cavipes, C. purpureus, C. rugosus, and C. violascens. Embryos of each species were cultured in vitro at 6 dif- ferent temperatures (18 to 34°C) in artificial seawater to avoid air desiccation; the lower threshold temperatures for embryonic development were estimated using heat summation theory equa- tions. Additionally, partial effective cumulative temperatures (> lower threshold temperature) until hatching were determined for ovigerous females of each species cultured in containers. The relationships between the embryonic growth index values (relative area of the embryonic body vs. total embryo surface) and effective cumulative temperatures were expressed using cubic equa- tions. Lower threshold temperature was estimated to be 12.7 to 14.5°C. The effective cumulative temperature and egg incubation period estimates from the appearance of the embryonic body to hatching were higher in B. latro and C. brevimanus, followed by C. rugosus, C. cavipes, and C. violascens, and lower in C. -

Diversity and Abundance of Intertidal Crabs at the East Swamp- Managed Areas in Segara-Anakan Cilacap, Central Java, Indonesia
Deutscher Tropentag, October 8-10, 2003, Göttingen “Technological and Institutional Innovations for Sustainable Rural Development” Diversity and Abundance of Intertidal Crabs at the East Swamp- Managed Areas in Segara-Anakan Cilacap, Central Java, Indonesia 1 2 2 MOH. HUSEIN SASTRANEGARA , HELEEN FERMON AND MICHAEL MÜHLENBERG 1 University of Jenderal Soedirman, Faculty of Biology, 53122 Purwokerto, Indonesia, e-mail: [email protected] 2 Georg August Universität Göttingen, Centre for Nature Conservation, 37075 Göttingen, Germany, e-mail: [email protected] 2 Georg August Universität Göttingen, Centre for Nature Conservation, 37075 Göttingen, Germany, e-mail: [email protected] Abstract Mangrove forests possess a high diversity and abundance of crabs in Central Java. The conservation of mangrove forests into prawn ponds causes depletion of supply of river sediments and loss of property. The main objective of this study is to compare the diversity and abundance of intertidal crabs in undisturbed, crab hunting, logging and prawn pond areas that were different in percent mangrove canopy covers and percent sediment textures. In each area, two transect lines were installed to analyse the percent mangrove canopy cover, and also the percent sediment texture comparing to trilinier plot. In total, 16,353 intertidal crab individuals in 13 species were sampled. Differences in observed number and estimated number of species (ACE, Chao1), as well as number of individuals, diversity indices and evenness between the four studied mangrove areas were all highly significant. Monthly fluctuation of intertidal crab diversity was more constant in the undisturbed area with a high mangrove coverage (90%) compared to the crab hunting, the logging and the prawn pond area with a coverage of 89%, 33% and 0%, respectively. -

Rearing Enhancement of Ovalipes Trimaculatus (Crustacea: Portunidae
www.nature.com/scientificreports OPEN Rearing enhancement of Ovalipes trimaculatus (Crustacea: Portunidae) zoea I by feeding on Artemia persimilis nauplii enriched with alternative microalgal diets Antonela Martelli1,3*, Elena S. Barbieri1,3, Jimena B. Dima2 & Pedro J. Barón1 The southern surf crab Ovalipes trimaculatus (de Haan, 1833) presents a high potential for aquaculture. In this study, we analyze the benefts of diferent dietary treatments on its molt success and ftness of larval stages. Artemia persimilis nauplii were enriched with monospecifc (Nannochloropsis oculata, Tetraselmis suecica, Dunaliella salina, Isochrysis galbana and Chaetoceros gracilis) and multispecifc (Mix) microalgal diets twice a day over a 48-h period. Mean total length (TL), growth instar number (I) and gut fullness rate (GFR) of nauplii showed signifcant diferences between dietary treatments at several sampling times, optimal results being observed in those providing Mix. Artemia nauplii grown under most experimental dietary treatments reached the capture size limit for Ovalipes trimaculatus zoea I (700 µm) within 24 h. After that time interval, Mix-enriched nauplii were amongst those with higher protein contents. Ovalipes trimaculatus zoea I fed on Artemia nauplii enriched during 24 h under diferent dietary treatments showed signifcant diferences in survival, inter-molt duration, molting success to zoea II and motility. Optimal results were observed in zoea I fed on Mix-enriched Artemia nauplii. This work not only represents a frst step towards the dietary optimization for O. trimaculatus zoeae rearing but also provides the frst results on the use of enriched A. persimilis. Portunid crabs stand out as highly valued resources for fsheries and aquaculture because of their export potential and high nutritional value1. -

Nutritional Composition, Antioxidants and Antimicrobial Activities In
Research Journal of Biotechnology Vol. 15 (4) April (2020) Res. J. Biotech Nutritional Composition, Antioxidants and Antimicrobial Activities in Muscle Tissues of Mud Crab, Scylla paramamosain Wan Roslina Wan Yusof1*, Noorasmin Mokhtar Ahmad2, Mohd Alhafiizh Zailani1, Mardhiah Mohd Shahabuddin1, Ngieng Ngui Sing2 and Awang Ahmad Sallehin Awang Husaini2 1. Centre for Pre-University Studies, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, MALAYSIA 2. Faculty of Resource Science and Technology, Universiti Malaysia Sarawak, 94300 Kota Samarahan, Sarawak, MALAYSIA *[email protected] Abstract paramamosain, Scylla serrata, Scylla transquebarica and Mud crab, Scylla paramamosain known as a green mud Scylla olivacea. Interestingly, S. paramamosain also known crab, has become a popular seafood due to its meat as a green mud crab is one of the popular species and widely quality. In addition, this marine invertebrate has been distributed in mangrove area with high water salinity such as continental coast of South China Sea and Java Sea.10 found to possess peptides with different biological activities and potentials. The aim was, first, to Among the other species, S. paramamosain has triangular determine the basic nutritional content and second, to frontal lobe spines and easily identified by the dotted pattern screen for the antioxidants and antimicrobials on its propodus. From nutritional point of view, mud crabs activities in the tissue of mud crab, S. paramamosain. have high protein, minerals and polyunsaturated fatty acids Percentages of carbohydrate, protein and fat in S. contents.23 Apart from nutritional view, many studies have paramamosain were 2.32%, 12.53% and 0.23% been conducted on the biological activities in mud crabs, respectively. -

A New Pathogenic Virus in the Caribbean Spiny Lobster Panulirus Argus from the Florida Keys
DISEASES OF AQUATIC ORGANISMS Vol. 59: 109–118, 2004 Published May 5 Dis Aquat Org A new pathogenic virus in the Caribbean spiny lobster Panulirus argus from the Florida Keys Jeffrey D. Shields1,*, Donald C. Behringer Jr2 1Virginia Institute of Marine Science, The College of William & Mary, Gloucester Point, Virginia 23062, USA 2Department of Biological Sciences, Old Dominion University, Norfolk, Virginia 23529, USA ABSTRACT: A pathogenic virus was diagnosed from juvenile Caribbean spiny lobsters Panulirus argus from the Florida Keys. Moribund lobsters had characteristically milky hemolymph that did not clot. Altered hyalinocytes and semigranulocytes, but not granulocytes, were observed with light microscopy. Infected hemocytes had emarginated, condensed chromatin, hypertrophied nuclei and faint eosinophilic Cowdry-type-A inclusions. In some cases, infected cells were observed in soft con- nective tissues. With electron microscopy, unenveloped, nonoccluded, icosahedral virions (182 ± 9 nm SD) were diffusely spread around the inner periphery of the nuclear envelope. Virions also occurred in loose aggregates in the cytoplasm or were free in the hemolymph. Assembly of the nucleocapsid occurred entirely within the nucleus of the infected cells. Within the virogenic stroma, blunt rod-like structures or whorls of electron-dense granular material were apparently associated with viral assembly. The prevalence of overt infections, defined as lethargic animals with milky hemolymph, ranged from 6 to 8% with certain foci reaching prevalences of 37%. The disease was transmissible to uninfected lobsters using inoculations of raw hemolymph from infected animals. Inoculated animals became moribund 5 to 7 d before dying and they began dying after 30 to 80 d post-exposure. -

Mud Crab Susceptibility to Disease from White Spot Syndrome Virus Is Species-Dependent Somboonna Et Al
Mud crab susceptibility to disease from white spot syndrome virus is species-dependent Somboonna et al. Somboonna et al. BMC Research Notes 2010, 3:315 http://www.biomedcentral.com/1756-0500/3/315 (20 November 2010) Somboonna et al. BMC Research Notes 2010, 3:315 http://www.biomedcentral.com/1756-0500/3/315 SHORT REPORT Open Access Mud crab susceptibility to disease from white spot syndrome virus is species-dependent Naraporn Somboonna1,2,5*, Seksan Mangkalanan3, Attasit Udompetcharaporn2, Chartchai Krittanai3, Kallaya Sritunyalucksana1,2, TW Flegel1,2,4 Abstract Background: Based on a report for one species (Scylla serrata), it is widely believed that mud crabs are relatively resistant to disease caused by white spot syndrome virus (WSSV). We tested this hypothesis by determining the degree of susceptibility in two species of mud crabs, Scylla olivacea and Scylla paramamosain, both of which were identified by mitochondrial 16 S ribosomal gene analysis. We compared single-dose and serial-dose WSSV challenges on S. olivacea and S. paramamosain. Findings: In a preliminary test using S. olivacea alone, a dose of 1 × 106 WSSV copies/g gave 100% mortality within 7 days. In a subsequent test, 17 S. olivacea and 13 S. paramamosain were divided into test and control groups for challenge with WSSV at 5 incremental, biweekly doses starting from 1 × 104 and ending at 5 × 106 copies/g. For 11 S. olivacea challenged, 3 specimens died at doses between 1 × 105 and 5 × 105 copies/g and none died for 2 weeks after the subsequent dose (1 × 106 copies/g) that was lethal within 7 days in the preliminary test. -

The Effect of Different Ph and Photoperiod Regimens on The
Short communication: The effect of different pH and photoperiod regimens on the survival rate and developmental period of the larvae of Portunus pelagicus (Decapoda, Brachyura, Portunidae) Item Type article Authors Ravi, R.; Manisseri, M.K. Download date 28/09/2021 09:20:50 Link to Item http://hdl.handle.net/1834/37376 Iranian Journal of Fisheries Sciences Short Communication 12(2) 490-499 2013 __________________________________________________________________________________________ The effect of different pH and photoperiod regimens on the survival rate and developmental period of the larvae of Portunus pelagicus (Decapoda, Brachyura, Portunidae) Ravi R.*; Manisseri M. K. Received: December 2011 Accepted: August 2012 -Marine Biodiversity Division, Central Marine Fisheries Research Institute, Post Box No. 1603, Kochi-682 018, India *Corresponding author’s e mail: [email protected] Keywords: Portunus pelagicus, Photoperiod, Larval rearing, Larval development, Larval survival. Crustacean larval development occurs (Waddy and Aiken, 1991), cannibalism within a narrow range of environmental (Hecht and Pienaar, 1993) and swimming parameters (Sastry, 1983). Among the activity and hence metabolism (Gardner, abiotic characteristics that influence the 1996). developmental period and survival rate of The effects of photoperiod on the larvae, pH and photoperiod are of great larval survival have been studied in several importance. High and low levels of pH are species such as the common shrimp known to adversely affect the physiology Crangon -
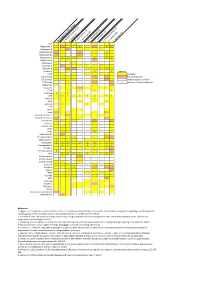
Neuropeptide Comparative Distribution.Xlsx
Allatostatin‐A 3 4 Allatostatin‐B Allatostatin‐B1 2 ACP Allatostatin‐B2 2 Allatostatin‐C 2 2 2 2 3 2 Allatostatin‐cc N. norgevicus (this study) Allatotropin C. quad ri carinatus (1) Bursicon‐A Bursicon‐B P. clark ii (2) Calcitonin 2 2 Legends CCHamide‐1H. ame Partial sequencesricanus (3) CCHamide‐2 CCAP S. verreauxi (4) CCRFamide n number of isoforms detected CNMamide M. rosenbergii (5) Corazonin CFSH‐likeCFSH 4 2 4 4 L. vann amei (6) CHH‐likeCHH 3 2 4 4 3 2 8 3 3 S. par amamosain (7) MIH‐like 2 2 Eclosion hormone 1 MIH 3 4 2 C. maenas (8) Eclosion hormone 2 E. sinensis (9) DH31 ITP DH44 C GSEFLamide C FMRFamideElevenin C C Kinin/LeucokininHIGSLYRamide 2 2 GPA2 MyosuppressinGPB5 6 2 C C Neuropeptide FNeuroparsin 2 2 3 2 3 2 3 2 3 4 2 5 3 3 Periviscerokinin 3 Orcokinin 2 Prohormone‐1 Prohormone‐3 Prohormone‐4 PDH 3 3 1 2 5 5 3 Available Undetected/not available Proctolin Pyrokinin 2 Relaxin RyamideRPCH Vasopressin‐neurophysin SIFamidesNPF 2 References Sulfakinin 1. Nguyen TV, Cummins SF, Elizur A, Ventura T. 2016. Transcriptomic characterization and curation of candidate neuropeptides regulating reproduction in the Tachykinin eyestalk ganglia of the Australian crayfish, 2. Veenstra JA. 2015. The power of next‐generation sequencing as illustrated by the neuropeptidome of the crayfish Procambarus clarkii. General and WXXXRamide Trissin comparative endocrinology 224: 84‐95. 3. Christie AE, Chi M, Lameyer TJ, Pascual MG, Shea DN, Stanhope ME, Schulz DJ, Dickinson PS. 2015. Neuropeptidergic signaling in the American lobster Homarus americanus 4. -

Neuropeptides in the Cerebral Ganglia of the Mud Crab, Scylla
www.nature.com/scientificreports OPEN Neuropeptides in the cerebral ganglia of the mud crab, Scylla paramamosain: transcriptomic Received: 30 May 2015 Accepted: 23 October 2015 analysis and expression profiles Published: 23 November 2015 during vitellogenesis Chenchang Bao1, Yanan Yang3, Huiyang Huang1 & Haihui Ye1,2 Neuropeptides play a critical role in regulating animal reproduction. In vertebrates, GnRH, GnIH and kisspeptin are the key neuropeptide hormones of the reproductive axis, however, the reproductive axis for invertebrates is vague. Knowledge on ovarian development of the mud crab, Scylla paramamosain, is critical for aquaculture and resources management of the commercially important species. This study employed Illumina sequencing, reverse transcription-polymerase chain reaction and quantitative real-time PCR techniques to identify neuropeptides that may be involved in ovarian development of S. paramamosain. A total of 32 neuropeptide transcripts from two dozen neuropeptide families, 100 distinct mature peptides were predicted from the transcriptome data of female S. paramamosain cerebral ganglia. Among them, two families, i.e. GSEFLamide and WXXXRamide, were first identified from the cerebral ganglia of crustaceans. Of these neuropeptides, 21 transcripts of interest were selected for further confirmation and all of them were detected in the cerebral ganglia, as well as in other nervous tissues and the ovary. Most of them also had differential expression in the cerebral ganglia during various vitellogenic stages, suggesting their likely involvement in regulating vitellogenesis and ovarian maturation. Overall, these findings provide an important basis for subsequent studies on peptide function in reproduction of S. paramamosain. Neuropeptides are ubiquitous in the nervous system of the animal kingdom from cnidarians to human1.