Nutritional Composition, Antioxidants and Antimicrobial Activities In
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ovarian Development of the Mud Crab Scylla Paramamosain in a Tropical Mangrove Swamps, Thailand
Available Online JOURNAL OF SCIENTIFIC RESEARCH Publications J. Sci. Res. 2 (2), 380-389 (2010) www.banglajol.info/index.php/JSR Ovarian Development of the Mud Crab Scylla paramamosain in a Tropical Mangrove Swamps, Thailand M. S. Islam1, K. Kodama2, and H. Kurokura3 1Department of Aquaculture and Fisheries, Jessore Science and Technology University, Jessore- 7407, Bangladesh 2Marine Science Institute, The University of Texas at Austin, Channel View Drive, Port Aransas, Texas 78373, USA 3Laboratory of Global Fisheries Science, Department of Global Agricultural Sciences, The University of Tokyo, Bunkyo, Tokyo 113-8657, Japan Received 15 October 2009, accepted in revised form 21 March 2010 Abstract The present study describes the ovarian development stages of the mud crab, Scylla paramamosain from Pak Phanang mangrove swamps, Thailand. Samples were taken from local fishermen between June 2006 and December 2007. Ovarian development was determined based on both morphological appearance and histological observation. Ovarian development was classified into five stages: proliferation (stage I), previtellogenesis (II), primary vitellogenesis (III), secondary vitellogenesis (IV) and tertiary vitellogenesis (V). The formation of vacuolated globules is the initiation of primary vitellogenesis and primary growth. The follicle cells were found around the periphery of the lobes, among the groups of oogonia and oocytes. The follicle cells were hardly visible at the secondary and tertiary vitellogenesis stages. Yolk granules occurred in the primary vitellogenesis stage and are first initiated in the inner part of the oocytes, then gradually concentrated to the periphery of the cytoplasm. The study revealed that the initiation of vitellogenesis could be identified by external observation of the ovary but could not indicate precisely. -

Growth Analysis, Mortality and Exploitation Level of Mud Crab
Jurnal Kelautan Tropis Maret 2020 Vol. 23(1):136-144 P-ISSN : 1410-8852 E-ISSN : 2528-3111 Growth analysis, mortality and exploitation level of Mud Crab Scylla serrata, Forskål 1775, (Malacostraca : Portunidae) in Mangkang Wetan waters, Semarang, Central Java, Indonesia Ervia Yudiati1,2*, Arumning Tias Fauziah1, Irwani1, Agus Setyawan3 and Insafitri4 1Department of Marine Sciences, Faculty of Fisheries and Marine Science, Diponegoro University Jl. Prof. Soedarto SH, Tembalang, Semarang 50275 2Laboratory of Marine Biotechnology, Diponegoro University 3Department of Fisheries and Marine Science, Faculty of Agriculture, Lampung University Jl. Soemantri Brojonegoro No 1, Bandar Lampung 35145 4Marine Science Programme Study, Faculty of Agriculture, Madura Trunojoyo University Jl. Raya Telang, Kamal, Bangkalan 69162 Email: [email protected] Abstract Awareness of Mud Crab over exploitation in Mangkang Wetan Waters has been noticed. One of the reference information is the growth study to determine the condition of the mud crab population. High demand encourages the fisherman to catch more, which leads to overexploitation in nature. The study aimed to estimate the growth, mortality, and exploitation rate of mud crabs. The 921 mud crabs samples were collected from Mangkang Wetan Waters from October 2018 to January 2019. The method used was the survey method. The crabs were taken once a week for 4 months. The width and weight of crab carapace were measured. The growth rate of S. serrata was 0.93/year (male) and 0.69/year (female). The natural mortality rate of S. serrata was 1.08/year (male) and 0.89/year (female), the mortality of catch (F) was 0.55/year (male) and 1.09/year (female). -

A Systematic and Experimental Analysis of Their Genes, Genomes, Mrnas and Proteins; and Perspective to Next Generation Sequencing
Crustaceana 92 (10) 1169-1205 CRUSTACEAN VITELLOGENIN: A SYSTEMATIC AND EXPERIMENTAL ANALYSIS OF THEIR GENES, GENOMES, MRNAS AND PROTEINS; AND PERSPECTIVE TO NEXT GENERATION SEQUENCING BY STEPHANIE JIMENEZ-GUTIERREZ1), CRISTIAN E. CADENA-CABALLERO2), CARLOS BARRIOS-HERNANDEZ3), RAUL PEREZ-GONZALEZ1), FRANCISCO MARTINEZ-PEREZ2,3) and LAURA R. JIMENEZ-GUTIERREZ1,5) 1) Sea Science Faculty, Sinaloa Autonomous University, Mazatlan, Sinaloa, 82000, Mexico 2) Coelomate Genomic Laboratory, Microbiology and Genetics Group, Industrial University of Santander, Bucaramanga, 680007, Colombia 3) Advanced Computing and a Large Scale Group, Industrial University of Santander, Bucaramanga, 680007, Colombia 4) Catedra-CONACYT, National Council for Science and Technology, CDMX, 03940, Mexico ABSTRACT Crustacean vitellogenesis is a process that involves Vitellin, produced via endoproteolysis of its precursor, which is designated as Vitellogenin (Vtg). The Vtg gene, mRNA and protein regulation involve several environmental factors and physiological processes, including gonadal maturation and moult stages, among others. Once the Vtg gene, mRNAs and protein are obtained, it is possible to establish the relationship between the elements that participate in their regulation, which could either be species-specific, or tissue-specific. This work is a systematic analysis that compares the similarities and differences of Vtg genes, mRNA and Vtg between the crustacean species reported in databases with respect to that obtained from the transcriptome of Callinectes arcuatus, C. toxotes, Penaeus stylirostris and P. vannamei obtained with MiSeq sequencing technology from Illumina. Those analyses confirm that the Vtg obtained from selected species will serve to understand the process of vitellogenesis in crustaceans that is important for fisheries and aquaculture. RESUMEN La vitelogénesis de los crustáceos es un proceso que involucra la vitelina, producida a través de la endoproteólisis de su precursor llamado Vitelogenina (Vtg). -

Zoologische Verhandelingen
CRUSTACEA LIBRARY SMITHSONIAN INST. RETURN TO W-119 ZOOLOGISCHE VERHANDELINGEN UITGEGEVEN DOOR HET RIJKSMUSEUM VAN NATUURLIJKE HISTORIE TE LEIDEN (MINISTERIE VAN CULTUUR, RECREATIE EN MAATSCHAPPELIJK WERK) No. 162 A COLLECTION OF DECAPOD CRUSTACEA FROM SUMBA, LESSER SUNDA ISLANDS, INDONESIA by L. B. HOLTHUIS LEIDEN E. J. BRILL 14 September 1978 ZOOLOGISCHE VERHANDELINGEN UITGEGEVEN DOOR HET RIJKSMUSEUM VAN NATUURLIJKE HISTORIE TE LEIDEN (MINISTERIE VAN CULTUUR, RECREATIE EN MAATSCHAPPELIJIC WERK) No. 162 A COLLECTION OF DECAPOD CRUSTACEA FROM SUMBA, LESSER SUNDA ISLANDS, INDONESIA by i L. B. HOLTHUIS LEIDEN E. J. BRILL 14 September 1978 Copyright 1978 by Rijksmuseum van Natuurlijke Historie, Leiden, The Netherlands All rights reserved. No part of this hook may he reproduced or translated in any form, by print, photoprint, microfilm or any other means without written permission from the publisher PRINTED IN THE NETHERLANDS A COLLECTION OF DECAPOD CRUSTACEA FROM SUMBA, LESSER SUNDA ISLANDS, INDONESIA by L. B. HOLTHUIS Rijksmuseum van Natuurlijke Historic, Leiden, Netherlands With 14 text-figures and 1 plate The Sumba-Expedition undertaken by Dr. E. Sutter of the Naturhistori- sches Museum of Basle and Dr. A. Biihler of the Museum fur Volkerkunde of the same town, visited the Lesser Sunda Islands, Indonesia, in 1949. Dr. Sutter, the zoologist, stayed in the islands from 19 May to 26 November; most of the time was spent by him in Sumba (21 May-31 October), and extensive collections were made there, among which a most interesting material of Decapod Crustacea, which forms the subject of the present paper. A few Crustacea were collected on the islands of Sumbawa (on 19 May) and Flores (19 and 21 November). -

Redalyc.Crecimiento Del Camarón Excavador Parastacus Pugnax
Latin American Journal of Aquatic Research E-ISSN: 0718-560X [email protected] Pontificia Universidad Católica de Valparaíso Chile Ibarra, Mauricio; Arana, Patricio M. Crecimiento del camarón excavador Parastacus pugnax (Poeppig, 1835) determinado mediante técnica de marcaje Latin American Journal of Aquatic Research, vol. 39, núm. 2, julio, 2011, pp. 378-384 Pontificia Universidad Católica de Valparaíso Valparaiso, Chile Disponible en: http://www.redalyc.org/articulo.oa?id=175019398018 Cómo citar el artículo Número completo Sistema de Información Científica Más información del artículo Red de Revistas Científicas de América Latina, el Caribe, España y Portugal Página de la revista en redalyc.org Proyecto académico sin fines de lucro, desarrollado bajo la iniciativa de acceso abierto Lat. Am. J. Aquat. Res., 39(2): 378-384, 2011 Lat. Am. J. Aquat. Res. 378 DOI: 10.3856/vol39-issue2-fulltext-18 Short Communication Crecimiento del camarón excavador Parastacus pugnax (Poeppig, 1835) determinado mediante técnica de marcaje Mauricio Ibarra1 & Patricio M. Arana1 1Escuela de Ciencias del Mar, Pontificia Universidad Católica de Valparaíso P.O. Box 1020, Valparaíso, Chile RESUMEN. Para determinar el crecimiento en el camarón excavador (Parastacus pugnax) en la zona centro sur de Chile se utilizó un marbete tipo cinturón. Los parámetros longitud cefalotorácica asintótica (Lc∞) y la velocidad de incremento en longitud y peso (K), se establecieron mediante el método de Gulland & Holt (1959). El parámetro t0 se determinó mediante la ecuación inversa del modelo de von Bertalanffy, estableciéndose que las curvas de crecimiento en longitud y peso fueron definidas por los parámetros K = 0,35 -1 mm año , t0 = -0,38 años, Lc∞ = 55,9 mm y W∞ = 83,8 g, valores similares a los de Samastacus spinifrons, especie chilena de alto potencial de cultivo, y de otros parastácidos sudamericanos, tales como P. -

The Mating Success and Hybridization of Mud Crab, Scylla Spp. in Controlled Tanks Gunarto, Sulaeman, Herlinah
The mating success and hybridization of Mud crab, Scylla spp. in controlled tanks Gunarto, Sulaeman, Herlinah Research Institute for Coastal Aquaculture and Fisheries Extension Maros, South Sulawesi, Indonesia. Corresponding author: Gunarto, [email protected] Abstract. Interspecific hybridization in mud crabs hardly occurs in uncontrolled conditions (in the wild). Therefore, the purpose of this study is to investigate the reproductive performance of female broodstock (fecundity, hatchability and crablet production) after mating with the same species and interspecific hybridization among Scylla spp. in controlled tanks. Four rounded fiberglass tanks, 1 m high and with a diameter of 2.1 m, were filled with 32 ppt saline filtered seawater. Then, 10 pairs (male/female) of mud crab broodstocks were stocked in each tank for mating and hybridization. The study involved four treatments: 1. Scylla paramamosain male paired with S. tranquebarica female; 2. S. tranquebarica male paired with S. paramamosain female; 3. S. tranquebarica male paired with the females of S. paramamosain, S. olivacea, and S. tranquebarica; 4. S. paramamosain male paired with females of S. tranquebarica, S. olivacea, and S. paramamosain. The number of precopulation and copulation incidences were recorded daily. Post copulated female crabs grew individually in different tanks until the gonads matured and the crabs spawned. The results of the research showed that the precopulation incidence obtained in treatment tanks 2, 3 and 4 were not significantly different (P>0.05), but they were significantly higher than the treatment in tank 1 (P<0.05). The interspecific hybridization between the female of S. paramamosain and the male of S. tranquebarica resulted in egg fecundities from 32200 to 1868000 eggs, and a hatching rate between 2 and 45%. -

Full Text in Pdf Format
Vol. 25: 83–96, 2016 AQUATIC BIOLOGY Published August 24 doi: 10.3354/ab00663 Aquat Biol OPENPEN ACCESSCCESS Thermal adaptations of embryos of six terrestrial hermit crab species Katsuyuki Hamasaki1,*, Takahiro Matsuda1, Ken Takano1, Mio Sugizaki1, Yu Murakami1, Shigeki Dan2, Shuichi Kitada1 1Graduate School of Marine Science and Technology, Tokyo University of Marine Science and Technology, Minato, Konan, Tokyo 108-8477, Japan 2Research Centre for Marine Invertebrate, National Research Institute of Fisheries and Environment of Inland Sea, Fisheries Research Agency, Momoshima, Onomichi, Hiroshima 722-0061, Japan ABSTRACT: We evaluated the thermal adaptations of embryos of 6 terrestrial hermit crab species in the family Coenobitidae (genera Birgus and Coenobita): B. latro, C. brevimanus, C. cavipes, C. purpureus, C. rugosus, and C. violascens. Embryos of each species were cultured in vitro at 6 dif- ferent temperatures (18 to 34°C) in artificial seawater to avoid air desiccation; the lower threshold temperatures for embryonic development were estimated using heat summation theory equa- tions. Additionally, partial effective cumulative temperatures (> lower threshold temperature) until hatching were determined for ovigerous females of each species cultured in containers. The relationships between the embryonic growth index values (relative area of the embryonic body vs. total embryo surface) and effective cumulative temperatures were expressed using cubic equa- tions. Lower threshold temperature was estimated to be 12.7 to 14.5°C. The effective cumulative temperature and egg incubation period estimates from the appearance of the embryonic body to hatching were higher in B. latro and C. brevimanus, followed by C. rugosus, C. cavipes, and C. violascens, and lower in C. -

Mud Crab Susceptibility to Disease from White Spot Syndrome Virus Is Species-Dependent Somboonna Et Al
Mud crab susceptibility to disease from white spot syndrome virus is species-dependent Somboonna et al. Somboonna et al. BMC Research Notes 2010, 3:315 http://www.biomedcentral.com/1756-0500/3/315 (20 November 2010) Somboonna et al. BMC Research Notes 2010, 3:315 http://www.biomedcentral.com/1756-0500/3/315 SHORT REPORT Open Access Mud crab susceptibility to disease from white spot syndrome virus is species-dependent Naraporn Somboonna1,2,5*, Seksan Mangkalanan3, Attasit Udompetcharaporn2, Chartchai Krittanai3, Kallaya Sritunyalucksana1,2, TW Flegel1,2,4 Abstract Background: Based on a report for one species (Scylla serrata), it is widely believed that mud crabs are relatively resistant to disease caused by white spot syndrome virus (WSSV). We tested this hypothesis by determining the degree of susceptibility in two species of mud crabs, Scylla olivacea and Scylla paramamosain, both of which were identified by mitochondrial 16 S ribosomal gene analysis. We compared single-dose and serial-dose WSSV challenges on S. olivacea and S. paramamosain. Findings: In a preliminary test using S. olivacea alone, a dose of 1 × 106 WSSV copies/g gave 100% mortality within 7 days. In a subsequent test, 17 S. olivacea and 13 S. paramamosain were divided into test and control groups for challenge with WSSV at 5 incremental, biweekly doses starting from 1 × 104 and ending at 5 × 106 copies/g. For 11 S. olivacea challenged, 3 specimens died at doses between 1 × 105 and 5 × 105 copies/g and none died for 2 weeks after the subsequent dose (1 × 106 copies/g) that was lethal within 7 days in the preliminary test. -

What Crab Is It?
What crab is it? Item Type article Authors Buendia, Romeo Y. Download date 02/10/2021 05:36:48 Link to Item http://hdl.handle.net/1834/35065 aquafarm news • shrimp culture What crab is it? By R Y B uendia The mud crab Scylla spp. of the Portunidae family is widely distributed throughout the Mud crab classification Indo-west Pacific region. They are consid ered an important seafood item due to their First reported as Cancer serratus (Forskal 1755), Portunus tranquibaricus esteemed delicacy, medicinal and high (Fabricius 1793), andScylla olivacea (Herbst 1796), de Haan in 1833 choose the market value (Kathirvel e t al. 1997). Re nam e Scylla serrata after a mythical Greek sea monster Scylla who lived in a cave cent studies showed that there is a large (BOBP 1992). A century later, Estampador in 1949 identified three species and a market for mud crab worldwide (Globefish subspecies. This, however, was revised by Keenan et al. in 1998. Below is a com 1995; Austrade 1996). In the Philippines, parison (Fortes 1999): the Department of Science and Technol ogy included mud crab in its list of “Ex Estampador (1949a) K eenan et al. (1998) port Winners” in aquaculture (Fortes 1999). S. serrata S. olivacea Locally known as king crab or giant S. oceanica S. serrata crab, the Scylla serrata species is preferred S. serrata var. param am osain S. paramamosain by crab farmers. "They grow bigger and S. tranquebarica S. tranquebarica faster, some reaching 1 kg in just six months," says Avelino Triño, a crab expert at SEAFDEC. -

Survival, Growth, and Nutrient Content of the Mud Crabs (Scylla Olivacea) Which Cultivated in Mangrove Area with Different Types of Feed 1Muhammad Y
Physiological response: survival, growth, and nutrient content of the mud crabs (Scylla olivacea) which cultivated in mangrove area with different types of feed 1Muhammad Y. Karim, 1Hasni Y. Azis, 2Muslimin, 3Akbar M. Tahya 1 Department of Aquaculture, Faculty of Marine Science and Fishery, Hasanuddin University, Makassar, South Sulawesi, Indonesia; 2 Pangkep State Polytechnic for Agriculture, Pangkep, South Sulawesi, Indonesia; 3 Balik Diwa Marine Technology University, Makassar, South Sulawesi, Indonesia. Corresponding author: M. Y. Karim, [email protected] Abstract. Cultivation of mangrove crabs for fattening can be carried out in mangrove area or known as a silvofishery system. This study aimed to determine the best types of feed for mangrove crab (Scylla olivacea) fattening which cultivated in the mangrove area. The research was conducted in the mangrove area of Pangkep Regency, South Sulawesi Province, Indonesia. The experimental animal used was male mangrove crabs (S. olivacea) with a weight of 250±10 g. The container used was a confinement that made from bamboo with length, width, and height of 1.0 x 1.0 x 1.0 m respectively, and placed in the mangrove area. Four types of feed used referred to treatment with a dose of 10% of crab’s body weight. The frequency of feeding was twice per day namely in the morning by 30% and in the afternoon 70%. The study used a complete randomized design (CRD) consisting of 4 treatments and 3 replications. The different types of feed used were: (A) trash fish, (B) mujair fish, (C) oysters, and (D) rice field snails. The data were analyzed using variance analysis (ANOVA) and the further test was performed using W- Tuckey test. -

The Effect of Different Ph and Photoperiod Regimens on The
Short communication: The effect of different pH and photoperiod regimens on the survival rate and developmental period of the larvae of Portunus pelagicus (Decapoda, Brachyura, Portunidae) Item Type article Authors Ravi, R.; Manisseri, M.K. Download date 28/09/2021 09:20:50 Link to Item http://hdl.handle.net/1834/37376 Iranian Journal of Fisheries Sciences Short Communication 12(2) 490-499 2013 __________________________________________________________________________________________ The effect of different pH and photoperiod regimens on the survival rate and developmental period of the larvae of Portunus pelagicus (Decapoda, Brachyura, Portunidae) Ravi R.*; Manisseri M. K. Received: December 2011 Accepted: August 2012 -Marine Biodiversity Division, Central Marine Fisheries Research Institute, Post Box No. 1603, Kochi-682 018, India *Corresponding author’s e mail: [email protected] Keywords: Portunus pelagicus, Photoperiod, Larval rearing, Larval development, Larval survival. Crustacean larval development occurs (Waddy and Aiken, 1991), cannibalism within a narrow range of environmental (Hecht and Pienaar, 1993) and swimming parameters (Sastry, 1983). Among the activity and hence metabolism (Gardner, abiotic characteristics that influence the 1996). developmental period and survival rate of The effects of photoperiod on the larvae, pH and photoperiod are of great larval survival have been studied in several importance. High and low levels of pH are species such as the common shrimp known to adversely affect the physiology Crangon -
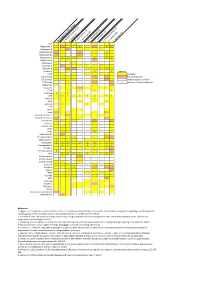
Neuropeptide Comparative Distribution.Xlsx
Allatostatin‐A 3 4 Allatostatin‐B Allatostatin‐B1 2 ACP Allatostatin‐B2 2 Allatostatin‐C 2 2 2 2 3 2 Allatostatin‐cc N. norgevicus (this study) Allatotropin C. quad ri carinatus (1) Bursicon‐A Bursicon‐B P. clark ii (2) Calcitonin 2 2 Legends CCHamide‐1H. ame Partial sequencesricanus (3) CCHamide‐2 CCAP S. verreauxi (4) CCRFamide n number of isoforms detected CNMamide M. rosenbergii (5) Corazonin CFSH‐likeCFSH 4 2 4 4 L. vann amei (6) CHH‐likeCHH 3 2 4 4 3 2 8 3 3 S. par amamosain (7) MIH‐like 2 2 Eclosion hormone 1 MIH 3 4 2 C. maenas (8) Eclosion hormone 2 E. sinensis (9) DH31 ITP DH44 C GSEFLamide C FMRFamideElevenin C C Kinin/LeucokininHIGSLYRamide 2 2 GPA2 MyosuppressinGPB5 6 2 C C Neuropeptide FNeuroparsin 2 2 3 2 3 2 3 2 3 4 2 5 3 3 Periviscerokinin 3 Orcokinin 2 Prohormone‐1 Prohormone‐3 Prohormone‐4 PDH 3 3 1 2 5 5 3 Available Undetected/not available Proctolin Pyrokinin 2 Relaxin RyamideRPCH Vasopressin‐neurophysin SIFamidesNPF 2 References Sulfakinin 1. Nguyen TV, Cummins SF, Elizur A, Ventura T. 2016. Transcriptomic characterization and curation of candidate neuropeptides regulating reproduction in the Tachykinin eyestalk ganglia of the Australian crayfish, 2. Veenstra JA. 2015. The power of next‐generation sequencing as illustrated by the neuropeptidome of the crayfish Procambarus clarkii. General and WXXXRamide Trissin comparative endocrinology 224: 84‐95. 3. Christie AE, Chi M, Lameyer TJ, Pascual MG, Shea DN, Stanhope ME, Schulz DJ, Dickinson PS. 2015. Neuropeptidergic signaling in the American lobster Homarus americanus 4.