Appendix B. Drug Codes Requiring Authorization the Following Codes Will Require Authorization for MA Effective Oct 1, 2017 Page
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2019 Aetna Pharmacy Drug Guide Aetna Premier Plus Plan
Plan for your best health 2019 Aetna Pharmacy Drug Guide Aetna Premier Plus Plan aetna.com 05.02.414.1 O (12/19) Aetna is the brand name used for products and services provided by one or more of the Aetna group of subsidiary companies, including Aetna Life Insurance Company and its affiliates (Aetna). Aetna Pharmacy Management refers to an internal business unit of Aetna Health Management, LLC. Aetna Pharmacy Management administers, but does not offer, insure or otherwise underwrite the prescription drug benefits portion of your health plan and has no financial responsibility therefor. 2019 Aetna Commercial Plan (Premier Plus) Table of Contents INFORMATIONAL SECTION..................................................................................................................7 *5-HT4 RECEPTOR AGONISTS*** - DRUGS FOR THE STOMACH................................................ 18 *ADHD/ANTI-NARCOLEPSY/ANTI-OBESITY/ANOREXIANTS* - DRUGS FOR THE NERVOUS SYSTEM.................................................................................................................................18 *AGENTS FOR NARCOTIC WITHDRAWAL*** - DRUGS FOR ADDICTION............................... 22 *AGENTS FOR OPIOID WITHDRAWAL*** - DRUGS FOR ADDICTION......................................22 *AMEBICIDES* - DRUGS FOR INFECTIONS.....................................................................................22 *AMINO ACIDS*** - DRUGS FOR NUTRITION................................................................................ 22 *AMINOGLYCOSIDES* - DRUGS FOR -

Use of Fluorescence to Guide Resection Or Biopsy of Primary Brain Tumors and Brain Metastases
Neurosurg Focus 36 (2):E10, 2014 ©AANS, 2014 Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases *SERGE MARBACHER, M.D., M.SC.,1,5 ELISABETH KLINGER, M.D.,2 LUCIA SCHWYZER, M.D.,1,5 INGEBORG FISCHER, M.D.,3 EDIN NEVZATI, M.D.,1 MICHAEL DIEPERS, M.D.,2,5 ULRICH ROELCKE, M.D.,4,5 ALI-REZA FATHI, M.D.,1,5 DANIEL COLUCCIA, M.D.,1,5 AND JAVIER FANDINO, M.D.1,5 Departments of 1Neurosurgery, 2Neuroradiology, 3Pathology, and 4Neurology, and 5Brain Tumor Center, Kantonsspital Aarau, Aarau, Switzerland Object. The accurate discrimination between tumor and normal tissue is crucial for determining how much to resect and therefore for the clinical outcome of patients with brain tumors. In recent years, guidance with 5-aminolev- ulinic acid (5-ALA)–induced intraoperative fluorescence has proven to be a useful surgical adjunct for gross-total resection of high-grade gliomas. The clinical utility of 5-ALA in resection of brain tumors other than glioblastomas has not yet been established. The authors assessed the frequency of positive 5-ALA fluorescence in a cohort of pa- tients with primary brain tumors and metastases. Methods. The authors conducted a single-center retrospective analysis of 531 patients with intracranial tumors treated by 5-ALA–guided resection or biopsy. They analyzed patient characteristics, preoperative and postoperative liver function test results, intraoperative tumor fluorescence, and histological data. They also screened discharge summaries for clinical adverse effects resulting from the administration of 5-ALA. Intraoperative qualitative 5-ALA fluorescence (none, mild, moderate, and strong) was documented by the surgeon and dichotomized into negative and positive fluorescence. -

BC Cancer Benefit Drug List September 2021
Page 1 of 65 BC Cancer Benefit Drug List September 2021 DEFINITIONS Class I Reimbursed for active cancer or approved treatment or approved indication only. Reimbursed for approved indications only. Completion of the BC Cancer Compassionate Access Program Application (formerly Undesignated Indication Form) is necessary to Restricted Funding (R) provide the appropriate clinical information for each patient. NOTES 1. BC Cancer will reimburse, to the Communities Oncology Network hospital pharmacy, the actual acquisition cost of a Benefit Drug, up to the maximum price as determined by BC Cancer, based on the current brand and contract price. Please contact the OSCAR Hotline at 1-888-355-0355 if more information is required. 2. Not Otherwise Specified (NOS) code only applicable to Class I drugs where indicated. 3. Intrahepatic use of chemotherapy drugs is not reimbursable unless specified. 4. For queries regarding other indications not specified, please contact the BC Cancer Compassionate Access Program Office at 604.877.6000 x 6277 or [email protected] DOSAGE TUMOUR PROTOCOL DRUG APPROVED INDICATIONS CLASS NOTES FORM SITE CODES Therapy for Metastatic Castration-Sensitive Prostate Cancer using abiraterone tablet Genitourinary UGUMCSPABI* R Abiraterone and Prednisone Palliative Therapy for Metastatic Castration Resistant Prostate Cancer abiraterone tablet Genitourinary UGUPABI R Using Abiraterone and prednisone acitretin capsule Lymphoma reversal of early dysplastic and neoplastic stem changes LYNOS I first-line treatment of epidermal -

Ophthalmologic Policy: Vascular Endothelial Growth Factor (VEGF) Inhibitors
UnitedHealthcare® Community Plan Medical Benefit Drug Policy Ophthalmologic Policy: Vascular Endothelial Growth Factor (VEGF) Inhibitors Policy Number: CS2021D0042S Effective Date: March 1, 2021 Instructions for Use Table of Contents Page Related Community Plan Policies Application.......................................................................................... 1 • Macular Degeneration Treatment Procedures Coverage Rationale ........................................................................... 1 • Maximum Dosage and Frequency Definitions ........................................................................................... 3 • Oncology Medication Clinical Coverage Applicable Codes .............................................................................. 3 Background ........................................................................................ 3 Commercial Policy Clinical Evidence .............................................................................23 • Ophthalmologic Policy: Vascular Endothelial Growth U.S. Food and Drug Administration ..............................................34 Factor (VEGF) Inhibitors Centers for Medicare and Medicaid Services .............................35 References .......................................................................................35 Policy History/Revision Information..............................................39 Instructions for Use .........................................................................39 Application This Medical Benefit -

Australian Public Assessment Report for Pegaspargase
Australian Public Assessment Report for pegaspargase Proprietary Product Name: Oncaspar Sponsor: Baxalta Australia September 2018 Therapeutic Goods Administration About the Therapeutic Goods Administration (TGA) • The Therapeutic Goods Administration (TGA) is part of the Australian Government Department of Health and is responsible for regulating medicines and medical devices. • The TGA administers the Therapeutic Goods Act 1989 (the Act), applying a risk management approach designed to ensure therapeutic goods supplied in Australia meet acceptable standards of quality, safety and efficacy (performance) when necessary. • The work of the TGA is based on applying scientific and clinical expertise to decision- making, to ensure that the benefits to consumers outweigh any risks associated with the use of medicines and medical devices. • The TGA relies on the public, healthcare professionals and industry to report problems with medicines or medical devices. TGA investigates reports received by it to determine any necessary regulatory action. • To report a problem with a medicine or medical device, please see the information on the TGA website < https://www.tga.gov.au>. About AusPARs • An Australian Public Assessment Report (AusPAR) provides information about the evaluation of a prescription medicine and the considerations that led the TGA to approve or not approve a prescription medicine submission. • AusPARs are prepared and published by the TGA. • An AusPAR is prepared for submissions that relate to new chemical entities, generic medicines, major variations and extensions of indications. • An AusPAR is a static document; it provides information that relates to a submission at a particular point in time. • A new AusPAR will be developed to reflect changes to indications and/or major variations to a prescription medicine subject to evaluation by the TGA. -

THE CONCISE GUIDE to PHARMACOLOGY 2017/18 Alexander, Stephen P
University of Dundee THE CONCISE GUIDE TO PHARMACOLOGY 2017/18 Alexander, Stephen P. H.; Fabbro, Doriano; Kelly, Eamonn; Marrion, Neil V.; Peters, John A.; Faccenda, Elena Published in: British Journal of Pharmacology DOI: 10.1111/bph.13876 Publication date: 2017 Licence: CC BY Document Version Publisher's PDF, also known as Version of record Link to publication in Discovery Research Portal Citation for published version (APA): Alexander, S. P. H., Fabbro, D., Kelly, E., Marrion, N. V., Peters, J. A., Faccenda, E., Harding, S. D., Pawson, A. J., Sharman, J. L., Southan, C., Davies, J. A., & CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Catalytic receptors. British Journal of Pharmacology, 174 (Suppl. 1), S225-S271. https://doi.org/10.1111/bph.13876 General rights Copyright and moral rights for the publications made accessible in Discovery Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from Discovery Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain. • You may freely distribute the URL identifying the publication in the public portal. Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 23. Sep. 2021 S.P.H. -

Aminolevulinic Acid (ALA) As a Prodrug in Photodynamic Therapy of Cancer
Molecules 2011, 16, 4140-4164; doi:10.3390/molecules16054140 OPEN ACCESS molecules ISSN 1420-3049 www.mdpi.com/journal/molecules Review Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer Małgorzata Wachowska 1, Angelika Muchowicz 1, Małgorzata Firczuk 1, Magdalena Gabrysiak 1, Magdalena Winiarska 1, Małgorzata Wańczyk 1, Kamil Bojarczuk 1 and Jakub Golab 1,2,* 1 Department of Immunology, Centre of Biostructure Research, Medical University of Warsaw, Banacha 1A F Building, 02-097 Warsaw, Poland 2 Department III, Institute of Physical Chemistry, Polish Academy of Sciences, 01-224 Warsaw, Poland * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel. +48-22-5992199; Fax: +48-22-5992194. Received: 3 February 2011 / Accepted: 3 May 2011 / Published: 19 May 2011 Abstract: Aminolevulinic acid (ALA) is an endogenous metabolite normally formed in the mitochondria from succinyl-CoA and glycine. Conjugation of eight ALA molecules yields protoporphyrin IX (PpIX) and finally leads to formation of heme. Conversion of PpIX to its downstream substrates requires the activity of a rate-limiting enzyme ferrochelatase. When ALA is administered externally the abundantly produced PpIX cannot be quickly converted to its final product - heme by ferrochelatase and therefore accumulates within cells. Since PpIX is a potent photosensitizer this metabolic pathway can be exploited in photodynamic therapy (PDT). This is an already approved therapeutic strategy making ALA one of the most successful prodrugs used in cancer treatment. Key words: 5-aminolevulinic acid; photodynamic therapy; cancer; laser; singlet oxygen 1. Introduction Photodynamic therapy (PDT) is a minimally invasive therapeutic modality used in the management of various cancerous and pre-malignant diseases. -

The Assessment of the Combined Treatment of 5-ALA Mediated Photodynamic Therapy and Thalidomide on 4T1 Breast Carcinoma and 2H11 Endothelial Cell Line
molecules Article The Assessment of the Combined Treatment of 5-ALA Mediated Photodynamic Therapy and Thalidomide on 4T1 Breast Carcinoma and 2H11 Endothelial Cell Line Krzysztof Zduniak, Katarzyna Gdesz-Birula, Marta Wo´zniak*, Kamila Du´s-Szachniewiczand Piotr Ziółkowski Department of Pathology, Wrocław Medical University, Marcinkowskiego 1, 50-368 Wrocław, Poland; [email protected] (K.Z.); [email protected] (K.G.-B.); [email protected] (K.D.-S.); [email protected] (P.Z.) * Correspondence: [email protected] or [email protected] Academic Editors: M. Amparo F. Faustino, Carlos J. P. Monteiro and Catarina I. V. Ramos Received: 29 September 2020; Accepted: 2 November 2020; Published: 7 November 2020 Abstract: Photodynamic therapy (PDT) is a low-invasive method of treatment of various diseases, mainly neoplastic conditions. PDT has been experimentally combined with multiple treatment methods. In this study, we tested a combination of 5-aminolevulinic acid (5-ALA) mediated PDT with thalidomide (TMD), which is a drug presently used in the treatment of plasma cell myeloma. TMD and PDT share similar modes of action in neoplastic conditions. Using 4T1 murine breast carcinoma and 2H11 murine endothelial cells lines as an experimental tumor model, we tested 5-ALA-PDT and TMD combination in terms of cytotoxicity, apoptosis, Vascular Endothelial Growth Factor (VEGF) expression, and, in 2H11 cells, migration capabilities by wound healing assay. We have found an enhancement of cytotoxicity in 4T1 cells, whereas, in normal 2H11 cells, this effect was not statistically significant. The addition of TMD decreased the production of VEGF after PDT in 2H11 cell line. -
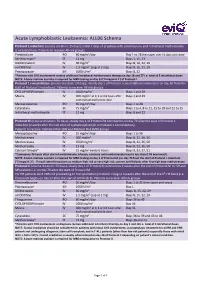
Acute Lymphoblastic Leukaemia: ALL06 Schema
Acute Lymphoblastic Leukaemia: ALL06 Schema Protocol 1 induction: (course duration: 35 days); initial 7 days of prephase with prednisolone and intrathecal methotrexate is included here. Patients to receive: All risk groups Prednisolone PO 60 mg/m2/day Day 1 to 28 then taper over 10 days and cease Methotrexate* IT 12 mg Days 1, 15, 33 DAUNOrubicin IV 30 mg/m2 Days 8, 16, 22, 29 vinCRISTine IV 1.5 mg/m2 (cap at 2 mg) Days 8, 16, 22, 29 Pegaspargase IM 1000 U/m2 Days 8, 22 *Patients with CNS involvement receive additional intrathecal methotrexate therapy on day 18 and 27 i.e. total of 5 intrathecal doses NOTE: A bone marrow aspirate is required for MRD testing on day 33 ('Timepoint 1') of Protocol I Protocol 1 consolidation: (course duration: 29 days); ideally day 1 of Protocol I consolidation commences on day 36 from the start of Protocol 1 induction ). Patients to receive: All risk groups. CYCLOPHOSPHamide IV 1000 mg/m2 Days 1 and 29 Mesna IV 400 mg/m2 at 0, 4 and 8 hours after Days 1 and 29 each cyclophosphamide dose Mercaptopurine PO 60 mg/m2/day Days 1 to 28 Cytarabine SC 75 mg/m2 Days 1 to 4, 8 to 11, 15 to 18 and 22 to 25 Intrathecal methotrexate IT 12 mg Days 8 and 22 Protocol M: (course duration: 56 days); ideally day 1 of Protocol M commences on day 79 after the start of Protocol 1 induction (2 weeks after the last dose of cyclophosphamide in Protocol 1 consolidation). -

Prodrugs: a Challenge for the Drug Development
PharmacologicalReports Copyright©2013 2013,65,1–14 byInstituteofPharmacology ISSN1734-1140 PolishAcademyofSciences Drugsneedtobedesignedwithdeliveryinmind TakeruHiguchi [70] Review Prodrugs:A challengeforthedrugdevelopment JolantaB.Zawilska1,2,JakubWojcieszak2,AgnieszkaB.Olejniczak1 1 InstituteofMedicalBiology,PolishAcademyofSciences,Lodowa106,PL93-232£ódŸ,Poland 2 DepartmentofPharmacodynamics,MedicalUniversityofLodz,Muszyñskiego1,PL90-151£ódŸ,Poland Correspondence: JolantaB.Zawilska,e-mail:[email protected] Abstract: It is estimated that about 10% of the drugs approved worldwide can be classified as prodrugs. Prodrugs, which have no or poor bio- logical activity, are chemically modified versions of a pharmacologically active agent, which must undergo transformation in vivo to release the active drug. They are designed in order to improve the physicochemical, biopharmaceutical and/or pharmacokinetic properties of pharmacologically potent compounds. This article describes the basic functional groups that are amenable to prodrug design, and highlights the major applications of the prodrug strategy, including the ability to improve oral absorption and aqueous solubility, increase lipophilicity, enhance active transport, as well as achieve site-selective delivery. Special emphasis is given to the role of the prodrug concept in the design of new anticancer therapies, including antibody-directed enzyme prodrug therapy (ADEPT) andgene-directedenzymeprodrugtherapy(GDEPT). Keywords: prodrugs,drugs’ metabolism,blood-brainbarrier,ADEPT,GDEPT -

Oncaspar, INN-Pegaspargase
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT Oncaspar 750 U/ml solution for injection/infusion. 2. QUALITATIVE AND QUANTITATIVE COMPOSITION One ml of solution contains 750 Units (U)** of pegaspargase*. One vial of 5 ml solution contains 3,750 Units. * The active substance is a covalent conjugate of Escherichia coli-derived L-asparaginase with monomethoxypolyethylene glycol **One unit is defined as the quantity of enzyme required to liberate 1 µmol ammonia per minute at pH 7.3 and 37°C The potency of this medicinal product should not be compared to the one of another pegylated or non-pegylated protein of the same therapeutic class. For more information, see section 5.1. For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Solution for injection/infusion. Clear, colourless solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Oncaspar is indicated as a component of antineoplastic combination therapy in acute lymphoblastic leukaemia (ALL) in paediatric patients from birth to 18 years, and adult patients. 4.2 Posology and method of administration Oncaspar should be prescribed and administered by physicians and/or health care personnel experienced in the use of antineoplastic products. It should only be given in a hospital setting where appropriate resuscitation equipment is available. Patients should be closely monitored for any adverse reactions throughout the administration period (see section 4.4). Posology Oncaspar is usually administered as part of combination chemotherapy protocols with other antineoplastic agents (see also section 4.5). Paediatric patients and adults ≤21 years The recommended dose in patients with a body surface area (BSA) ≥0.6 m2 and who are ≤21 years of age is 2,500 U of pegaspargase (equivalent to 3.3 ml Oncaspar)/m2 body surface area every 14 days. -

Quantum of Effectiveness Evidence in FDA's Approval of Orphan Drugs
Quantum of Effectiveness Evidence in FDA’s Approval of Orphan Drugs Cataloguing FDA’s Flexibility in Regulating Therapies for Persons with Rare Disorders by Frank J. Sasinowski, M.S., M.P.H., J.D.1 Chairman of the Board National Organization for Rare Disorders One of the key underlying issues facing the development of effective for their intended uses. all drugs, and particularly orphan drugs, is what kind of evi- dence the Food and Drug Administration (FDA) requires for FDA has for many decades acknowledged that there is a need approval. The Federal Food, Drug, and Cosmetic [FD&C] Act IRUÁH[LELOLW\LQDSSO\LQJLWVVWDQGDUGIRUDSSURYDO)RUH[- provides that for FDA to grant approval for a new drug, there ample, one of FDA’s regulations states that: “FDA will ap- must be “substantial evidence” of effectiveness derived from prove an application after it determines that the drug meets the “adequate and well-controlled investigations.” This language, statutory standards for safety and effectiveness… While the which dates from 1962, provides leeway for FDA medical re- statutory standards apply to all drugs, the many kinds of drugs viewers to make judgments as to what constitutes “substantial that are subject to the statutory standards and the wide range HYLGHQFHµRIDGUXJ·VHIIHFWLYHQHVVWKDWLVRILWVEHQHÀWWR RIXVHVIRUWKRVHGUXJVGHPDQGÁH[LELOLW\LQDSSO\LQJWKHVWDQ- patients. GDUGV7KXV)'$LVUHTXLUHGWRH[HUFLVHLWVVFLHQWLÀFMXGJPHQW to determine the kind and quantity of data and information an 7KHVROHODZWKDWDSSOLHVVSHFLÀFDOO\WRRUSKDQGUXJVWKH2U- applicant is required to provide for a particular drug to meet SKDQ'UXJ$FWRISURYLGHGÀQDQFLDOLQFHQWLYHVIRUGUXJ the statutory standards.” 21 C.F.R. § 314.105(c). FRPSDQLHVWRGHYHORSRUSKDQGUXJVZKLFKLVOHJDOO\GHÀQHG as products that treat diseases that affect 200,000 or fewer )'$SXEOLFO\KDVH[SUHVVHGVHQVLWLYLW\WRDSSO\LQJWKLVÁH[- patients in the U.S.