Basicranial Influence on Overall Cranial Shape
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Middle Cranial Fossa Sphenoidal Region Dural Arteriovenous Fistulas: Anatomic and Treatment Considerations
ORIGINAL RESEARCH INTERVENTIONAL Middle Cranial Fossa Sphenoidal Region Dural Arteriovenous Fistulas: Anatomic and Treatment Considerations Z.-S. Shi, J. Ziegler, L. Feng, N.R. Gonzalez, S. Tateshima, R. Jahan, N.A. Martin, F. Vin˜uela, and G.R. Duckwiler ABSTRACT BACKGROUND AND PURPOSE: DAVFs rarely involve the sphenoid wings and middle cranial fossa. We characterize the angiographic findings, treatment, and outcome of DAVFs within the sphenoid wings. MATERIALS AND METHODS: We reviewed the clinical and radiologic data of 11 patients with DAVFs within the sphenoid wing that were treated with an endovascular or with a combined endovascular and surgical approach. RESULTS: Nine patients presented with ocular symptoms and 1 patient had a temporal parenchymal hematoma. Angiograms showed that 5 DAVFs were located on the lesser wing of sphenoid bone, whereas the other 6 were on the greater wing of the sphenoid bone. Multiple branches of the ICA and ECA supplied the lesions in 7 patients. Four patients had cortical venous reflux and 7 patients had varices. Eight patients were treated with transarterial embolization using liquid embolic agents, while 3 patients were treated with transvenous embo- lization with coils or in combination with Onyx. Surgical disconnection of the cortical veins was performed in 2 patients with incompletely occluded DAVFs. Anatomic cure was achieved in all patients. Eight patients had angiographic and clinical follow-up and none had recurrence of their lesions. CONCLUSIONS: DAVFs may occur within the dura of the sphenoid wings and may often have a presentation similar to cavernous sinus DAVFs, but because of potential associations with the cerebral venous system, may pose a risk for intracranial hemorrhage. -

MR Imaging of the Orbital Apex
J Korean Radiol Soc 2000;4 :26 9-0 6 1 6 MR Imaging of the Orbital Apex: An a to m y and Pat h o l o g y 1 Ho Kyu Lee, M.D., Chang Jin Kim, M.D.2, Hyosook Ahn, M.D.3, Ji Hoon Shin, M.D., Choong Gon Choi, M.D., Dae Chul Suh, M.D. The apex of the orbit is basically formed by the optic canal, the superior orbital fis- su r e , and their contents. Space-occupying lesions in this area can result in clinical d- eficits caused by compression of the optic nerve or extraocular muscles. Even vas c u l a r changes in the cavernous sinus can produce a direct mass effect and affect the orbit ap e x. When pathologic changes in this region is suspected, contrast-enhanced MR imaging with fat saturation is very useful. According to the anatomic regions from which the lesions arise, they can be classi- fied as belonging to one of five groups; lesions of the optic nerve-sheath complex, of the conal and intraconal spaces, of the extraconal space and bony orbit, of the cav- ernous sinus or diffuse. The characteristic MR findings of various orbital lesions will be described in this paper. Index words : Orbit, diseases Orbit, MR The apex of the orbit is a complex region which con- tains many nerves, vessels, soft tissues, and bony struc- Anatomy of the orbital apex tures such as the superior orbital fissure and the optic canal (1-3), and is likely to be involved in various dis- The orbital apex region consists of the optic nerve- eases (3). -

Nasal Morphology and Its Correlation to Craniofacial Morphology in Lateral Cephalometric Analysis
International Journal of Environmental Research and Public Health Article Nasal Morphology and Its Correlation to Craniofacial Morphology in Lateral Cephalometric Analysis Agnieszka Jankowska 1 , Joanna Janiszewska-Olszowska 2,* and Katarzyna Grocholewicz 2 1 Private Practice “Dental Clinic Jankowscy”, 68-200 Zary,˙ Poland; [email protected] 2 Department of Interdisciplinary Dentistry, Pomeranian Medical University in Szczecin, 70-111 Szczecin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-91-466-1690 Abstract: Nose shape, size, and inclination influence facial appearance, but few studies concern the relationship between the nasal profile and craniofacial structures. The objective of this study was to analyze association of nasal cephalometric variables with skeletal structures, age, and sex. Cephalometric and nasal analysis was performed in 386 Polish orthodontic patients (aged 9–25 years). Student t-test and Mann–Whitney test were used to compare quantitative variables and Pearson’s or Spearman’s correlation coefficients—to find correlations. Soft tissue facial convexity angle corre- lates to Holdaway ratio, ANB (A-Nasion-B), and Wits appraisal. Nasal dorsum axis, nose length, nose depth (1) and nose depth (2), nose hump, lower dorsum convexity, and columella convexity increase with age. Nasal base angle, nasolabial angle, nasomental angle, soft tissue facial convex- ity and nasal bone angle decrease with age. Nasal base angle and nasomental angle are smaller in females. Thus, a relationship exists between nasal morphology and sagittal jaw configuration. Nasal parameters significantly change with age. Sexual dimorphism characterizes nasal bone angle Citation: Jankowska, A.; and nasomental angle. Janiszewska-Olszowska, J.; Grocholewicz, K. Nasal Morphology Keywords: nose; nose profile; cephalometry; orthodontics and Its Correlation to Craniofacial Morphology in Lateral Cephalometric Analysis. -

Craniotomy for Anterior Cranial Fossa Meningiomas: Historical Overview
Neurosurg Focus 36 (4):E14, 2014 ©AANS, 2014 Craniotomy for anterior cranial fossa meningiomas: historical overview SAUL F. MORALES-VALERO, M.D., JAMIE J. VAN GOMPEL, M.D., IOANNIS LOUMIOTIS, M.D., AND GIUSEPPE LANZINO, M.D. Department of Neurologic Surgery, Mayo Clinic, Mayo Medical School, Rochester, Minnesota The surgical treatment of meningiomas located at the base of the anterior cranial fossa is often challenging, and the evolution of the surgical strategy to resect these tumors parallels the development of craniotomy, and neurosur- gery in general, over the past century. Early successful operations to treat these tumors were pioneered by prominent figures such as Sir William Macewen and Francesco Durante. Following these early reports, Harvey Cushing made significant contributions, allowing a better understanding and treatment of meningiomas in general, but particularly those involving the anterior cranial base. Initially, large-sized unilateral or bilateral craniotomies were necessary to approach these deep-seated lesions. Technical advances such as the introduction of electrosurgery, the operating microscope, and refined microsurgical instruments allowed neurosurgeons to perform less invasive surgical proce- dures with better results. Today, a wide variety of surgical strategies, including endoscopic surgery and radiosurgery, are used to treat these tumors. In this review, the authors trace the evolution of craniotomy for anterior cranial fossa meningiomas. (http://thejns.org/doi/abs/10.3171/2014.1.FOCUS13569) KEY WORDS • intracranial meningiomas • craniotomy • history • anterior cranial fossa ENINGIOMAS of the anterior cranial fossa represent has a few distinct clinical features. However, in practice, 12%–20% of all intracranial meningiomas.5,30 this group of tumors often represents a continuum. -

Ardipithecus Ramidus and the Evolution of the Human Cranial Base
Ardipithecus ramidus and the evolution of the human cranial base William H. Kimbela,1, Gen Suwab, Berhane Asfawc, Yoel Raka,d, and Tim D. Whitee,1 aInstitute of Human Origins and School of Human Evolution and Social Change, Arizona State University, Tempe, AZ 85287; bThe University Museum, University of Tokyo, Bunkyo-ku, Tokyo 113-0033, Japan; cRift Valley Research Service, Addis Ababa, Ethiopia; dDepartment of Anatomy and Anthropology, Sackler School of Medicine, Tel Aviv University, 69978 Ramat Aviv, Israel; and eDepartment of Integrative Biology, Human Evolution Research Center, University of California, Berkeley, CA 94720 Contributed by Tim D. White, December 5, 2013 (sent for review October 14, 2013) The early Pliocene African hominoid Ardipithecus ramidus was di- We report here results of a metrical and morphological study agnosed as a having a unique phylogenetic relationship with the of the Ar. ramidus basicranium as another test of its hypothesized Australopithecus + Homo clade based on nonhoning canine teeth, phylogenetic affinity with Australopithecus and Homo. We ana- a foreshortened cranial base, and postcranial characters related to lyzed the length and breadth of the external cranial base and the facultative bipedality. However, pedal and pelvic traits indicating structural relationship between the petrous and tympanic elements substantial arboreality have raised arguments that this taxon may of the temporal bone in Ar. ramidus, Australopithecus (including instead be an example of parallel evolution of human-like traits Paranthropus of some authors), and mixed-sex samples of extant among apes around the time of the chimpanzee–human split. Here African hominoid (Gorilla gorilla, Pan troglodytes, Pan paniscus) we investigated the basicranial morphology of Ar. -

Neurocranial Histomorphometrics
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2012 Neurocranial Histomorphometrics Lindsay Hines Trammell University of Tennessee-Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Biological and Physical Anthropology Commons Recommended Citation Trammell, Lindsay Hines, "Neurocranial Histomorphometrics. " PhD diss., University of Tennessee, 2012. https://trace.tennessee.edu/utk_graddiss/1359 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Lindsay Hines Trammell entitled "Neurocranial Histomorphometrics." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Anthropology. Murray K. Marks, Major Professor We have read this dissertation and recommend its acceptance: Joanne L. Devlin, David A. Gerard, Walter E. Klippel, David G. Anderson (courtesy member) Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 5-2012 Neurocranial Histomorphometrics Lindsay Hines Trammell University of Tennessee-Knoxville, [email protected] This Dissertation is brought to you for free and open access by the Graduate School at Trace: Tennessee Research and Creative Exchange. -

Morfofunctional Structure of the Skull
N.L. Svintsytska V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 Ministry of Public Health of Ukraine Public Institution «Central Methodological Office for Higher Medical Education of MPH of Ukraine» Higher State Educational Establishment of Ukraine «Ukranian Medical Stomatological Academy» N.L. Svintsytska, V.H. Hryn Morfofunctional structure of the skull Study guide Poltava 2016 2 LBC 28.706 UDC 611.714/716 S 24 «Recommended by the Ministry of Health of Ukraine as textbook for English- speaking students of higher educational institutions of the MPH of Ukraine» (minutes of the meeting of the Commission for the organization of training and methodical literature for the persons enrolled in higher medical (pharmaceutical) educational establishments of postgraduate education MPH of Ukraine, from 02.06.2016 №2). Letter of the MPH of Ukraine of 11.07.2016 № 08.01-30/17321 Composed by: N.L. Svintsytska, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor V.H. Hryn, Associate Professor at the Department of Human Anatomy of Higher State Educational Establishment of Ukraine «Ukrainian Medical Stomatological Academy», PhD in Medicine, Associate Professor This textbook is intended for undergraduate, postgraduate students and continuing education of health care professionals in a variety of clinical disciplines (medicine, pediatrics, dentistry) as it includes the basic concepts of human anatomy of the skull in adults and newborns. Rewiewed by: O.M. Slobodian, Head of the Department of Anatomy, Topographic Anatomy and Operative Surgery of Higher State Educational Establishment of Ukraine «Bukovinian State Medical University», Doctor of Medical Sciences, Professor M.V. -

Descriptive Analysis of Human Remains from the Fuller and Fanning Mounds
AN ABSTRACT OF THE THESIS OF David Stepp for the degree of Master of Arts in Interdisciplinary Studies in the co- departments of Anthropology, Zoology, and Statistics presented onFebruary 2, 1994. Title :Descriptive Analysis of Human Remains from the Fuller and Fanning Mounds, Yamhill River, Willamette Valley, Oregon Redacted for Privacy Abstract Approved : Roberta Hall The study presents the results of a descriptive analysis of the skeletal remains of 66 individuals recovered from the Fuller and Fanning Mound sites, located on the Yamhill River, Willamette Valley, Oregon, excavated in 1941-42 by W. T. Edmundson and William S. Laughlin. The literature and original field notes have been analyzed, and a description of burial type, side, orientation, grave type, associations, original preservation, and other information has been compiled for each individual. A tally of each of these burial attributes for the Yamhill population as a whole is also completed. In addition, an assessment of age, sex, and stature, a series of craniometric measurements, and non-metric traits, a dental analysis, and general description of obvious pathologic and morphologic condition of each individual and the group as a whole have been accomplished. Differences in trade item associations between deformed and non-deformed individuals suggest either a later arrival of cranial deformation practices (and possibly another cultural group) to the area, and possibly a multiple occupation of the Fuller and Fanning sites, or an elite class separation defined in part by artificial deformation of crania. Cranial deformation is also associated with the frequency of certain cranial discrete traits. Sexual dimorphism was noted in metric but not in non-metric analyses. -

Morphology of the Foramen Magnum in Young Eastern European Adults
Folia Morphol. Vol. 71, No. 4, pp. 205–216 Copyright © 2012 Via Medica O R I G I N A L A R T I C L E ISSN 0015–5659 www.fm.viamedica.pl Morphology of the foramen magnum in young Eastern European adults F. Burdan1, 2, J. Szumiło3, J. Walocha4, L. Klepacz5, B. Madej1, W. Dworzański1, R. Klepacz3, A. Dworzańska1, E. Czekajska-Chehab6, A. Drop6 1Department of Human Anatomy, Medical University of Lublin, Lublin, Poland 2St. John’s Cancer Centre, Lublin, Poland 3Department of Clinical Pathomorphology, Medical University of Lublin, Lublin, Poland 4Department of Anatomy, Collegium Medicum, Jagiellonian University, Krakow, Poland 5Department of Psychiatry and Behavioural Sciences, Behavioural Health Centre, New York Medical College, Valhalla NY, USA 6Department of General Radiology and Nuclear Medicine, Medical University of Lublin, Lublin, Poland [Received 21 July 2012; Accepted 7 September 2012] Background: The foramen magnum is an important anatomical opening in the base of the skull through which the posterior cranial fossa communicates with the vertebral canal. It is also related to a number of pathological condi- tions including Chiari malformations, various tumours, and occipital dysplasias. The aim of the study was to evaluate the morphology of the foramen magnum in adult individuals in relation to sex. Material and methods: The morphology of the foramen magnum was evalu- ated using 3D computer tomography images in 313 individuals (142 male, 171 female) aged 20–30 years. Results: The mean values of the foramen length (37.06 ± 3.07 vs. 35.47 ± ± 2.60 mm), breadth (32.98 ± 2.78 vs. 30.95 ± 2.71 mm) and area (877.40 ± ± 131.64 vs. -
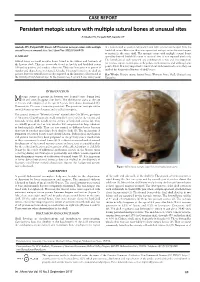
Persistent Metopic Suture with Multiple Sutural Bones at Unusual Sites
CASE REPORT Persistent metopic suture with multiple sutural bones at unusual sites Ambade HV, Fulpatil MP, Kasote AP Ambade HV, Fulpatil MP, Kasote AP. Persistent metopic suture with multiple in a human skull at asterion, left pterion and right coronal suture apart from the sutural bones at unusual sites. Int J Anat Var. 2017;10(3):69-70. lambdoid suture. Moreover, there was a persistent metopic suture between bregma to nasion in the same skull. The metopic suture with multiple sutural bones SUMMARY spreading beyond lambdoid suture at unusual sites is not reported previously. The knowledge of such variation and combination is rare and very important Sutural bones are small irregular bones found in the sutures and fontanels of for forensic expert, radiologists, orthopedists, neurosurgeons and anthropologist the human skull. They are commonly found at lambda and lambdoid suture point of view. It is very important to know about such variation because they can followed by pterion; and rarely at other sites. They vary from person to person in mislead the diagnosis of fracture of skull bones. number and shape, hence not named. Usually, 1-3 sutural bones in one skull are present, but 8-10 sutural bones are also reported in the literature, all restricted in Key Words: Metopic suture; Sutural bones; Wormian bones; Skull; Unusual sites; the vicinity of lambdoid sutures. In the present case, 8 sutural bones were present Variations INTRODUCTION etopic suture is present in between two frontal bones during fetal Mlife and soon disappear after birth. The obliteration starts at the age of 2 years and completed at the age of 8 years from above downwards (1). -
Lab Manual Axial Skeleton Atla
1 PRE-LAB EXERCISES When studying the skeletal system, the bones are often sorted into two broad categories: the axial skeleton and the appendicular skeleton. This lab focuses on the axial skeleton, which consists of the bones that form the axis of the body. The axial skeleton includes bones in the skull, vertebrae, and thoracic cage, as well as the auditory ossicles and hyoid bone. In addition to learning about all the bones of the axial skeleton, it is also important to identify some significant bone markings. Bone markings can have many shapes, including holes, round or sharp projections, and shallow or deep valleys, among others. These markings on the bones serve many purposes, including forming attachments to other bones or muscles and allowing passage of a blood vessel or nerve. It is helpful to understand the meanings of some of the more common bone marking terms. Before we get started, look up the definitions of these common bone marking terms: Canal: Condyle: Facet: Fissure: Foramen: (see Module 10.18 Foramina of Skull) Fossa: Margin: Process: Throughout this exercise, you will notice bold terms. This is meant to focus your attention on these important words. Make sure you pay attention to any bold words and know how to explain their definitions and/or where they are located. Use the following modules to guide your exploration of the axial skeleton. As you explore these bones in Visible Body’s app, also locate the bones and bone markings on any available charts, models, or specimens. You may also find it helpful to palpate bones on yourself or make drawings of the bones with the bone markings labeled. -

The Skull O Neurocranium, Form and Function O Dermatocranium, Form
Lesson 15 ◊ Lesson Outline: ♦ The Skull o Neurocranium, Form and Function o Dermatocranium, Form and Function o Splanchnocranium, Form and Function • Evolution and Design of Jaws • Fate of the Splanchnocranium ♦ Trends ◊ Objectives: At the end of this lesson, you should be able to: ♦ Describe the structure and function of the neurocranium ♦ Describe the structure and function of the dermatocranium ♦ Describe the origin of the splanchnocranium and discuss the various structures that have evolved from it. ♦ Describe the structure and function of the various structures that have been derived from the splanchnocranium ♦ Discuss various types of jaw suspension and the significance of the differences in each type ◊ References: ♦ Chapter: 9: 162-198 ◊ Reading for Next Lesson: ♦ Chapter: 9: 162-198 The Skull: From an anatomical perspective, the skull is composed of three parts based on the origins of the various components that make up the final product. These are the: Neurocranium (Chondocranium) Dermatocranium Splanchnocranium Each part is distinguished by its ontogenetic and phylogenetic origins although all three work together to produce the skull. The first two are considered part of the Cranial Skeleton. The latter is considered as a separate Visceral Skeleton in our textbook. Many other morphologists include the visceral skeleton as part of the cranial skeleton. This is a complex group of elements that are derived from the ancestral skeleton of the branchial arches and that ultimately gives rise to the jaws and the skeleton of the gill