Rare Occurrence of Reinhardt's Cranch Squid Liocranchia Reinhardti
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Feeding Habits and Feeding Grounds of the Northern Elephant Seal Richard
FEEDING HABITS AND FEEDING GROUNDS OF THE NORTHERN ELEPHANT SEAL RICHARDCONDIT AND BUHNEYJ, LE BOEUF Department of Biology, University of California, Santa Cruz, CA 95064 ABSTRAC’r-Prey species consumed by northern elephant seals were identified from the stom- ach and throat contents of dead seals and from observations of prey captured. Their diet is catholic, consisting of a variety of pelagic, deep water squid, Pacific hake, sharks, rays, and ratfish. Feeding grounds of elephant seals were inferred from sightings of tagged elephant seals at non-rookery locations. Feeding areas extended from northern Baja California to northern Vancouver Island. Juveniles of both sexes and adult males moved north from their haul out sites in search of food, travelling furthest north during the summer. A few sightings suggested that adult females remain in the vicinity of the rookeries where they breed. Northern elephant seals, Mirounga angustirostris, breed and molt in large aggregations on land in Baja California and California, but spend the majority of the year feeding at sea. The large breeding aggregations are easy to observe and a great deal is known about the elephant seal’s reproductive behavior (Le Boeuf, 1974; Reiter et al., 1981). In contrast, the animals are rarely observed at sea and little is known about their feeding biology. Existing information on the food habits of the northern elephant seal comes from the exam- ination of stomach contents of only nine specimens (Huey, 1930; Freiberg and Dumas, 1954; Cowan and Guiguet, 1956; Morejohn and Baltz, 1970; Antonelis and Fiscus, 1980; Jones, 1981). The remains of sharks, ratfish, squids, and bony fish were identified. -

Forage Fish Management Plan
Oregon Forage Fish Management Plan November 19, 2016 Oregon Department of Fish and Wildlife Marine Resources Program 2040 SE Marine Science Drive Newport, OR 97365 (541) 867-4741 http://www.dfw.state.or.us/MRP/ Oregon Department of Fish & Wildlife 1 Table of Contents Executive Summary ....................................................................................................................................... 4 Introduction .................................................................................................................................................. 6 Purpose and Need ..................................................................................................................................... 6 Federal action to protect Forage Fish (2016)............................................................................................ 7 The Oregon Marine Fisheries Management Plan Framework .................................................................. 7 Relationship to Other State Policies ......................................................................................................... 7 Public Process Developing this Plan .......................................................................................................... 8 How this Document is Organized .............................................................................................................. 8 A. Resource Analysis .................................................................................................................................... -

Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1
Pacific Science (1995), vol. 49, no. 2: 143-155 © 1995 by University of Hawai'i Press. All rights reserved Aspects of the Natural History of Pelagic Cephalopods of the Hawaiian Mesopelagic-Boundary Region 1 RICHARD EDWARD YOUNG 2 ABSTRACT: Pelagic cephalopods of the mesopelagic-boundary region in Hawai'i have proven difficult to sample but seem to occupy a variety ofhabitats within this zone. Abralia trigonura Berry inhabits the zone only as adults; A. astrosticta Berry may inhabit the inner boundary zone, and Pterygioteuthis giardi Fischer appears to be a facultative inhabitant. Three other mesopelagic species, Liocranchia reinhardti (Steenstrup), Chiroteuthis imperator Chun, and Iridoteuthis iris (Berry), are probable inhabitants; the latter two are suspected to be nonvertical migrants. The mesopelagic-boundary region also contains a variety of other pelagic cephalopods. Some are transients, common species of the mesopelagic zone in offshore waters such as Abraliopsis spp., neritic species such as Euprymna scolopes Berry, and oceanic epipelagic species such as Tremoctopus violaceus Chiaie and Argonauta argo Linnaeus. Others are appar ently permanent but either epipelagic (Onychoteuthis sp. C) or demersal (No totodarus hawaiiensis [Berry] and Haliphron atlanticus Steenstrup). Submersible observations show that Nototodarus hawaiiensis commonly "sits" on the bot tom and Haliphron atlanticus broods its young in the manner of some pelagic octopods. THE CONCEPT OF the mesopelagic-boundary over bottom depths of the same range. The region (m-b region) was first introduced by designation of an inner zone is based on Reid et al. (1991), although a general asso Reid'sfinding mesopelagic fishes resident there ciation of various mesopelagic animals with during both the day and night; mesopelagic land masses has been known for some time. -

A Generic Revision of the Cranchiidae (Cephalopoda; Oegopsida)
BULLETIN OF MARINE SCIENCE, 30(2): 365-412, 1980 A GENERIC REVISION OF THE CRANCHIIDAE (CEPHALOPODA; OEGOPSIDA) Nancy A. Voss ABSTRACT This paper is the first in a monographic study of the cephalopod family Cranchiidae. Thirteen of the 41 nominal genera are recognized as valid for the family: Cranchia, Lio- cmnchia, Leachia, He/icocranchia, Bathothauma, Sanda/ops, Ligllriella, Taonius, Ga/iteu- this, Mesonychoteuthis, Egea, Megalocmllchia and Teuthowenia. A full definition is given for the family and each valid genus; diagnoses are given for the two subfamilies Cranchiinae and Taoniinae. A generic key is accompanied by illustrations of a representative species of each genus. A comprehensive historical resume for the family is given and generic interre- lationships are discussed. The subfamily Taoniinae comprises roughly three groups of genera based primarily on the development and relationships of the posterior end of the gladius (Ianceola and conus) and the fins and on the photophore pattern on the eye. He/icocrallchia, Bathothauma, Sandalops and Liguriella form a loose group of the more primitive genera that possess a short conus (lost in Bathothallma) and small subterminal or terminal fins. Taollills, Galitellthis and Me- sOllychoteuthis form a group of specialized genera in which the conus and associated terminal fins are elongate and the large suckers of the club are modified into hooks or suckers with one or two large, central, hook-like teeth. Of these, Ga/itellthis and Mesollychoteuthi.\· are closely related. In the third group formed of Egea, Megalocranchia and Teuthowenia, the fins elongate and simultaneously extend up the lateral margins of the mantle as the conus elongates. -

An Illustrated Key to the Families of the Order
CLYDE F. E. ROP An Illustrated RICHARD E. YOl and GILBERT L. VC Key to the Families of the Order Teuthoidea Cephalopoda) SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • 1969 NUMBER 13 SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY NUMBER 13 Clyde F. E. Roper, An Illustrated Key 5K?Z" to the Families of the Order Teuthoidea (Cephalopoda) SMITHSONIAN INSTITUTION PRESS CITY OF WASHINGTON 1969 SERIAL PUBLICATIONS OF THE SMITHSONIAN INSTITUTION The emphasis upon publications as a means of diffusing knowledge was expressed by the first Secretary of the Smithsonian Institution. In his formal plan for the Institution, Joseph Henry articulated a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge not strictly professional." This keynote of basic research has been adhered to over the years in the issuance of thousands of titles in serial publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Annals of Flight Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in History and Technology In these series, the Institution publishes original articles and monographs dealing with the research and collections of its several museums and offices and of professional colleagues at other institutions of learning. These papers report newly acquired facts, synoptic interpretations of data, or original theory in specialized fields. -

Headed Whales (Peponocephala Electra) in Hawaiian Waters
Notes MARINE MAMMAL SCIENCE, 00(00): 00–00 (Month 2018) VC 2018 Society for Marine Mammalogy DOI: 10.1111/mms.12507 Stomach contents and diel diving behavior of melon-headed whales (Peponocephala electra) in Hawaiian waters 1 KRISTI L. WEST, Department of Human Nutrition, Food and Animal Science, College of Tropical Agriculture and Human Resources, Agricultural Sciences 216, 1955 East-West Road, University of Hawai‘i at Manoa, Honolulu, Hawai‘i 96822, U.S.A.; WILLIAM A. WALKER, Marine Mammal Laboratory, National Marine Fisheries Service, NOAA, 7600 Sand Point Way N.E., Seattle, Washington 98115, U.S.A.; ROBIN W. BAIRD,DANIEL L. WEBSTER, and GREGORY S. SCHORR, Cascadia Research Collective, 218 1=2 W. 4th Avenue, Olympia, Washington 98501, U.S.A. Knowledge of the diet and diving behavior of a species is crucial for understand- ing its behavior and ecology, and also has relevance to assessing the impact of poten- tial changes in behavior or spatial use. Assessing diet for many species of cetaceans is difficult, given that most foraging occurs far below the surface and that stomach contents of stranded animals are rarely available. Very little information on food habits or the diving behavior of melon-headed whales (Peponocephala electra)isavail- able from any region of the world. Although there is a paucity of knowledge on melon-headed whales, more is known about them in Hawaiian waters than anywhere else in the world (Aschettino et al. 2012, Woodworth et al. 2012, Baird 2016). In Hawai‘i, two populations of melon-headed whales are recognized, a Hawaiian Islands population estimated to be close to 5,000 individuals that travels offshore and among the islands, and a smaller, inshore population estimated to be about 450 individuals that is found off Hawai‘i Island and known as the Kohala resident population (Aschettino 2010, Aschettino et al. -

Defensive Behaviors of Deep-Sea Squids: Ink Release, Body Patterning, and Arm Autotomy
Defensive Behaviors of Deep-sea Squids: Ink Release, Body Patterning, and Arm Autotomy by Stephanie Lynn Bush A dissertation submitted in partial satisfaction of the requirements for the degree of Doctor of Philosophy in Integrative Biology in the Graduate Division of the University of California, Berkeley Committee in Charge: Professor Roy L. Caldwell, Chair Professor David R. Lindberg Professor George K. Roderick Dr. Bruce H. Robison Fall, 2009 Defensive Behaviors of Deep-sea Squids: Ink Release, Body Patterning, and Arm Autotomy © 2009 by Stephanie Lynn Bush ABSTRACT Defensive Behaviors of Deep-sea Squids: Ink Release, Body Patterning, and Arm Autotomy by Stephanie Lynn Bush Doctor of Philosophy in Integrative Biology University of California, Berkeley Professor Roy L. Caldwell, Chair The deep sea is the largest habitat on Earth and holds the majority of its’ animal biomass. Due to the limitations of observing, capturing and studying these diverse and numerous organisms, little is known about them. The majority of deep-sea species are known only from net-caught specimens, therefore behavioral ecology and functional morphology were assumed. The advent of human operated vehicles (HOVs) and remotely operated vehicles (ROVs) have allowed scientists to make one-of-a-kind observations and test hypotheses about deep-sea organismal biology. Cephalopods are large, soft-bodied molluscs whose defenses center on crypsis. Individuals can rapidly change coloration (for background matching, mimicry, and disruptive coloration), skin texture, body postures, locomotion, and release ink to avoid recognition as prey or escape when camouflage fails. Squids, octopuses, and cuttlefishes rely on these visual defenses in shallow-water environments, but deep-sea cephalopods were thought to perform only a limited number of these behaviors because of their extremely low light surroundings. -

Diversity of Cephalopoda from the Waters Around Taiwan
Phuket Marine Biological Center Special Publication 18(2): 331-340. (1998) 331 DIVERSITY OF CEPHALOPODA FROM THE WATERS AROUND TAIWAN C.C.Lu Department ofZoology, National Chung Hsing University, Taichung, Taiwan ABSTRACT Based on a new collection of cephalopods made during the period January 1995 to date, the list of cephalopods known to occur in Taiwanese waters, including the Taiwan Strait, has increased from 32 species to 64 species, belonging to 28 genera, 14 families, including sev eral species of sepiids and octopods new to science. The most speciose families are the family Sepiidae with 15 species, Loliginidae with 7 species, and Octopodidae with 22 spe cies.The fauna is largely in common with that of the neighbouring areas ofthe East China Sea and the South China Sea. When comparing the present result with previous reports, it is evident that the proportion of newly recorded taxa is large. At least 30 out ofthe 64 valid taxa reported are new records (46.9 %) and only 33 out of 63 nominal species previously reported are valid (52.4 %). As the present study is still in its early phase, it is expected that more taxa will be found when more habitats are sampled. Evidently our current knowl edge of cephalopod fauna of the area does not reflect the true diversity. Reasons for this disparity are examined. Using Taiwan as an example, recommendations are made to im prove our knowledge of the cephalopod fauna of the TMMP (Tropical Marine Mollusc Pro gramme) area. INTRODUCTION In China and Taiwan, cephalopods are tra in 19 genera belonging to nine families from ditionally used and prized as food items with Taiwanese waters. -

Identification and Estimation of Size from the Beaks of 18 Species of Cephalopods from the Pacific Ocean
17 NOAA Technical Report NMFS 17 Identification and Estimation of Size From the Beaks of 18 Species of Cephalopods From the Pacific Ocean Gary A. Wolff November 1984 U.S. DEPARTMENT OF COMMERCE National Oceanic and Atmospheric Administration National Marine Fisheries Service NOAA TECHNICAL REPORTS NMFS The major responsibilities of the National Marine Fisheries Service (NMFS) are to monitor and assess the abundance and geographic distribution of fishery resources, to understand and predict fluctuations in the quantity and distribution of these resources, and to establish levels for optimum use of the resources. NMFS is also charged with the development and implemen tation of policies for managing national fishing grounds, development and enforcement of domestic fisheries regulations, surveillance of foreign fishing off United States coastal waters, and the development and enforcement of international fishery agreements and policies. NMFS also assists the fishing industry through marketing service and economic analysis programs, and mortgage insurance and vessel construction subsidies. It collects, analyzes, and publishes statistics on various phases of the industry. The NOAA Technical Report NMFS series was established in 1983 to replace two subcategories of the Technical Reports series: "Special Scientific Report-Fisheries" and "Circular." The series contains the following types of reports: Scientific investigations that document long-term continuing programs of NMFS, intensive scientific reports on studies of restricted scope, papers on applied fishery problems, technical reports of general interest intended to aid conservation and management, reports that review in considerable detail and at a high technical level certain broad areas of research, and technical papers originating in economics studies and from management investigations. -
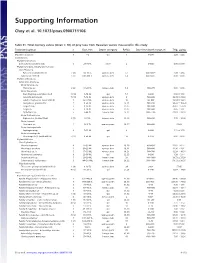
Supporting Information
Supporting Information Choy et al. 10.1073/pnas.0900711106 Table S1. Total mercury values (mean ؎ SD) of prey taxa from Hawaiian waters measured in this study Taxonomic group n Size, mm Depth category Ref(s). Day-time depth range, m THg, g/kg Mixed Zooplankton 5 1–2 epi 1 0–200 2.26 Ϯ 3.23 Invertebrates Phylum Ctenophora Ctenophores (unidentified) 3 20–30 TL other 2 0–600 0.00 Ϯ 0.00 Phylum Chordata, Subphylum Tunicata Class Thaliacea Pyrosomes (unidentified) 2 (8) 14–36 TL upmeso.dvm 2, 3 400–600ϩ 3.49 Ϯ 4.94 Salps (unidentified) 3 (7) 200–400 TL upmeso.dvm 2, 4 400–600ϩ 0.00 Ϯ 0.00 Phylum Arthropoda Subphylum Crustacea Order Amphipoda Phronima sp. 2 (6) 17–23 TL lomeso.dvm 5, 6 400–975 0.00 Ϯ 0.00 Order Decapoda Crab Megalopae (unidentified) 3 (14) 3–14 CL epi 7, 8 0–200 0.94 Ϯ 1.63 Janicella spinacauda 7 (10) 7–16 CL upmeso.dvm 9 500–600 30.39 Ϯ 23.82 Lobster Phyllosoma (unidentified) 5 42–67 CL upmeso.dvm 10 80–400 18.54 Ϯ 13.61 Oplophorus gracilirostris 5 9–20 CL upmeso.dvm 9, 11 500–650 90.23 Ϯ 103.20 Sergestes sp. 5 8–25 CL upmeso.dvm 12, 13 200–600 45.61 Ϯ 51.29 Sergia sp. 5 6–10 CL upmeso.dvm 12, 13 300–600 0.45 Ϯ 1.01 Systellapsis sp. 5 5–44 CL lomeso.dvm 9, 12 600–1100 22.63 Ϯ 38.18 Order Euphausiacea Euphausiids (unidentified) 2 (7) 5–7 CL upmeso.dvm 14, 15 400–600 7.72 Ϯ 10.92 Order Isopoda Anuropus sp. -

Giant Protistan Parasites on the Gills of Cephalopods (Mollusca)
DISEASES OF AQUATIC ORGANISMS Vol. 3: 119-125. 1987 Published December 14 Dis. aquat. Org. Giant protistan parasites on the gills of cephalopods (Mollusca) Norman ~c~ean',F. G. ~ochberg~,George L. shinn3 ' Biology Department, San Diego State University, San Diego, California 92182-0057, USA Department of Invertebrate Zoology, Santa Barbara Museum of Natural History, 2559 Puesta Del Sol Road, Santa Barbara, California 93105. USA Division of Science, Northeast Missouri State University, Kirksville. Missouri 63501, USA ABSTRACT: Large Protista of unknown taxonomic affinities are described from 3 species of coleoid squids, and are reported from many other species of cephalopods. The white to yellow-orange, ovoid cyst-like parasites are partially embedded within small pockets on the surface of the gills, often in large numbers. Except for a holdfast region on one side of the large end, the surface of the parasite is elaborated into low triangular plates separated by grooves. The parasites are uninucleate; their cytoplasm bears lipid droplets and presumed paraglycogen granules. Trichocysts, present in a layer beneath the cytoplasmic surface, were found by transmission electron microscopy to be of the dino- flagellate type. Further studies are needed to clarify the taxonomic position of these protists. INTRODUCTION epoxy resin (see below). One specimen each of the coleoid squids Abralia trigonura and Histioteuthis dof- Cephalopods harbor a diversity of metazoan and leini were trawled near Oahu, Hawaii, in March, 1980. protozoan parasites (Hochberg 1983). In this study we Gill parasites from the former were fixed in formalin; used light and electron microscopy to characterize a those from the latter were fixed in osmium tetroxide. -

A Review of Direct and Indirect Impacts of Marine Dredging Activities on Marine Mammals
A review of direct and indirect impacts of marine dredging activities on marine mammals Family Scientific name Common name Range of best Frequency of Minimum Methodology Diet Region Habitat Documented Effects of Potential Effects of Dredging (excluding (including hearing (10 dB minimum hearing Dredging subspecies) subspecies) from max; kHz) hearing threshold (dB threshold (kHz) re 1 µPa) Otariidae Arctocephalus Cape & Unknown; — — — Fish (e.g. Emmelichthys nitidus, F, J (Kirkman et Continental shelf waters (IUCN, — Habitat destruction, increase in pusillus Australian fur fundamental Pseudophycis bachus, Trachurus al., 2007; IUCN, 2012) turbidity, changes to prey seal frequency of declivis, Neoplatycephalus 2013; Perrin, availability, masking, incidental male in air barks Richardsoni) (Australian fur seal) 2013) capture or injury, avoidance & is 0.14 & female (Page et al., 2005) an increase in shipping traffic in air barks is 0.15 (Tripovich et al., 2008) Arctophoca Antarctic fur seal Unknown; peak — — — Fish (e.g. Gymnoscopelus A, F, J (IUCN, Forage in deep waters (>500 m) — Habitat destruction, increase in gazella frequency of in piabilis, Electrona subaspera, 2013; Perrin, with a strong chlorophyll turbidity, changes to prey air barks is 0.3– Champsocephalus gunnari) 2013; Reeves et concentration & steep availability, masking, incidental 5.9 (Page et al., (Guinet et al., 2001) al., 2002) bathymetric gradients, otherwise capture or injury, avoidance & 2002) remains close to the colony in an increase in shipping traffic areas with Polar