Effect of Thermal Treatment on the Cation Exchange and Disordering in Tourmaline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Povondraite. a Redefinition of the Tourmaline Ferridravite Jonr, D
American Mineralogist, Volume 78, pages 433-436, 1993 Povondraite. a redefinition of the tourmaline ferridravite Jonr, D. Gnrcn, T. Scorr Encrr Mineral SciencesSection, Canadian Museum of Nature, Ottawa, Ontario K1P 6P4, Canada FnaNx C. HlwrHoRNE Department of Geological Sciences,University of Manitoba, Winnipeg, Manitoba R3T 2N2, Canada Assrnlcr Povondraite, previously the tourmaline ferridravite, is redefined. It is rhombohedral, R3m, with a: 16.186(2)and c:7.444\l) A. fne new chemicalformula, derived by crystal structure analysis, is (NaoroKo ru),, ou (Fel.jrFeS j,Mgo rr)",o, (FeljrMg, .uAlo.r)r,nr- B.Si5e6O2,88(OH)r,,and the ideal end-memberformula is NaFel*Fel+(BO.).(SiuO,')- (o,oH)4. INrnooucrroN nm). Thesevalues are greaterthan those given by Walen- (1979). During the investigation of a seriesof tourmaline crys- ta and Dunn model tal structureswith varying contents of Fe and Mg (Grice Povondraitewas chemicallyanalyzed using a Jeol and Ercit, 1990, 1993) the "ferridravite" structurewas 733 electronmicroprobe. Wavelength-dispersionanaly- 15 kV, refined. It was discovered that the formula proposed by sesused an operatingvoltage of a beam current Walenta and Dunn (1979) for the speciesferridravite was of 25 nA measuredwith a Faradaycup, and a beam di- pm. were incorrect. They adopted the standard schemefor assign- ameter of l0 The following standards used: gehlenite(Al), (Si, ment of cationsto the X, Y, and Z sites,i.e., with Fert synthetic almandine Mg), synthetic (Fe),microcline (K), amphibole (Na), VP,O, and Al3* assignedto the Z site and Mg2* and Fe2+to the fayalite sodic (V), (Ti). were Y site, yielding the empirical formula (NaoroKoro)- and titanite The elementsF, Mn, and Ca sought but not detected. -

Fluor-Tsilaisite, Namn3al6(Si6o18)(BO3)
Mineralogical Magazine, February 2015, Vol. 79(1), pp. 89–101 Fluor-tsilaisite, NaMn3Al6(Si6O18)(BO3)3(OH)3F, a new tourmaline from San Piero in Campo (Elba, Italy) and new data on tsilaisitic tourmaline from the holotype specimen locality 1,2, 1,2 3 3 FERDINANDO BOSI *, GIOVANNI B. ANDREOZZI ,GIOVANNA AGROSI` AND EUGENIO SCANDALE 1 Dipartimento di Scienze della Terra, Sapienza Universita` di Roma, P. le Aldo Moro, 5, I-00185 Rome, Italy 2 CNR- Istituto di Geoscienze e Georisorse, VOS Roma, P. le Aldo Moro, 5, I-00185 Rome, Italy 3 Dipartimento di Scienze della Terra e Geoambientali, Universita` di Bari - Campus, via E. Orabona 4, I-70125 Bari, Italy [Received 30 April 2014; Accepted 10 July 2014; Associate Editor: S. Krivovichev] ABSTRACT Fluor-tsilaisite, NaMn3Al6(Si6O18)(BO3)3(OH)3F, is a new mineral of the tourmaline supergroup. It occurs in an aplitic dyke of a LCT-type pegmatite body from Grotta d’Oggi, San Piero in Campo, Elba Island, Italy, in association with quartz, K-feldspar, plagioclase, elbaite, schorl, fluor-elbaite and tsilaisite. Crystals are greenish yellow with a vitreous lustre, sub-conchoidal fracture and white streak. Fluor-tsilaisite has a Mohs hardness of ~7 and a calculated density of 3.134 g/cm3. In plane-polarized light, fluor-tsilaisite is pleochroic (O = pale greenish yellow and E = very pale greenish yellow), uniaxial negative. Fluor-tsilaisite is rhombohedral, space group R3m, a = 15.9398(6), c = 7.1363(3) A˚ , V = 1570.25(11) A˚ 3, Z = 3. The crystal structure of fluor-tsilaisite was refined to R1 = 3.36% using 3496 unique reflections collected with MoKa X-ray intensity data. -

Detrital Tourmaline As an Indicator of Provenance
Louisiana State University LSU Digital Commons LSU Master's Theses Graduate School 2003 Detrital tourmaline as an indicator of provenance: a chemical and sedimentological study of modern sands from the Black Hills, South Dakota David Brent Viator Louisiana State University and Agricultural and Mechanical College Follow this and additional works at: https://digitalcommons.lsu.edu/gradschool_theses Part of the Earth Sciences Commons Recommended Citation Viator, David Brent, "Detrital tourmaline as an indicator of provenance: a chemical and sedimentological study of modern sands from the Black Hills, South Dakota" (2003). LSU Master's Theses. 1520. https://digitalcommons.lsu.edu/gradschool_theses/1520 This Thesis is brought to you for free and open access by the Graduate School at LSU Digital Commons. It has been accepted for inclusion in LSU Master's Theses by an authorized graduate school editor of LSU Digital Commons. For more information, please contact [email protected]. DETRITAL TOURMALINE AS AN INDICATOR OF PROVENANCE: A CHEMICAL AND SEDIMENTOLOGICAL STUDY OF MODERN SANDS FROM THE BLACK HILLS, SOUTH DAKOTA A Thesis Submitted to the Graduate Faculty of Louisiana State University and Agricultural and Mechanical College in partial fulfillment of the requirements for the degree of Master of Science in The Department of Geology and Geophysics by David Brent Viator B.S., Louisiana State University, 1999 August, 2003 ACKNOWLEDGEMENTS I must first thank my major advisor, Dr. Darrell Henry, whose guidance has benefited me throughout my undergraduate and graduate studies. His patient mentoring has carried me from my undergraduate senior project through my graduate thesis. His contribution to my development as a geologist cannot be underestimated, and his efforts are greatly appreciated. -

Mg, Fe)Si2o6 (Mg, Fe) Sio4
INTERNAL GEODYNAMICS (ENDOGENOUS PROCESSES OF THE EARTH) İÇ JEODİNAMİK (DÜNYANIN ENDOJEN OLAYLARI) A. M. C. Şengör İTÜ Avrasya Yerbilimleri Enstitüsü 2005 Kış yarıyılı Lesson 4: The Minerals and Rocks of the Earth Part IIIa: The minerals- special mineralogy Because minerals show much greater variability in terms of their chemical composition than in terms of their crystal structure, it has been customary to classify them primarily according to chemical composition. The following classes have been recognised on the basis of the dominant anion or groups of anions: 1. Native elements 2. Sulfides Of all these classes of minerals only 3. Sulfosalts the silicates and the carbonates are 4. Oxides really important as rock-makers. All 5. Halides other minerals occur only as small 6. Carbonates contributors to the building of the 7. Nitrates earth’s crust or mantle. Only in the 8. Borates core, Fe-alloys assume a critical rôle. 9. Phosphates 10. Sulfates Let us remember the contribution of 11. Tungstates various minerals to the make-up of 12. Silicates the earth’s crust: The composition of the earth’s crust (after Ronov and Yaroshevsky, 1969; from Klein, 2002) The crust makes up about 0.03839 of the total earth mass Important message: The silicates are the most important minerals for our understanding of by far most of the geological phenomena. Of the silicates, the feldspars are the most important minerals for our understanding of the earth’s crust (both continental and oceanic). Therefore we must know them well. Some important feldspar references for this class: Janecke, S. U. and Evans, J. -

3O, a New Mineral from the Tourmaline Supergroup
American Mineralogist, Volume 98, pages 1886–1892, 2013 Darrellhenryite, Na(LiAl2)Al6(BO3)3Si6O18(OH)3O, a new mineral from the tourmaline supergroup MILAN NOVÁK1,*, ANDREAS ERTL2, PAVEL POVONDRA3, MICHAELA Vašinová GALIOVÁ4,5, GEORGE R. ROSSMAN6, HELMUT PRISTACZ2, MARKUS PREM2, GERALD GIESTER2, PETR GADAS1 1 AND RADEK šKODA 1Department of Geological Sciences, Masaryk University, Kotlářská 2, 611 37 Brno, Czech Republic 2Institut für Mineralogie und Kristallographie, Geozentrum, Universität Wien, Althanstrasse 14, 1090 Wien, Austria 3Department of Geochemistry, Mineralogy, and Natural Resources, Charles University, Albertov 6, 128 43 Praha 2, Czech Republic 4Department of Chemistry, Masaryk University, Kotlářská 2, 611 37 Brno, Czech Republic 5Central European Institute of Technology (CEITEC), Masaryk University, Kamenice 5, 625 00 Brno, Czech Republic 6Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125-2500, U.S.A. ABSTRACT Darrellhenryite, Na(LiAl2)Al6(BO3)3Si6O18(OH)3O, a new member of the tourmaline supergroup (related to the alkali-subgroup 4), is a new Li-bearing tourmaline species, which is closely related to Y W Y W elbaite through the substitution Al0.5O1Li –0.5(OH)–1. It occurs in a complex (Li-bearing) petalite-subtype pegmatite with common lepidolite, Li-bearing tourmalines, and amblygonite at Nová Ves near Český Krumlov, southern Bohemia, Moldanubian Zone, Czech Republic. This zoned pegmatite dike cross-cuts a serpentinite body enclosed in leucocratic granulites. Pink darrellhenryite forms columnar crystals (sometimes in parallel arrangement) up to 3 cm long and up 2 cm thick, associated with albite (var. cleavelandite), minor quartz, K-feldspar, petalite, rare polylithionite, and locally rare pollucite. -

Tourmaline the Indicator Mineral: from Atomic Arrangement to Viking
Tourmaline the Indicator Mineral: From Atomic Arrangement to Viking Navigation Tourmaline as a constituent of jewelry. FROM THE TOURMALINE 1 2 COLLECTION OF Frank C. Hawthorne and Dona M. Dirlam DR. E. SOKOLOVA 1811-5209/11/0007-0307$2.50 DOI: 10.2113/gselements.7.5.307 ourmaline sensu lato has been known for at least two thousand years, and its unique combination of physical properties has ensured its impor- Ttance to human society, from technical devices (such as a possible Viking navigational aid and early piezoelectric gauges in the 20th century) to attractive and popular gemstones. The chemical diversity and accommo- dating nature of its structure combine to make tourmaline a petrogenetic indicator for the wide range of rocks in which it occurs. Recent advances in understanding the structure, site assignments, and substitution mechanisms have led to a new nomenclature for the tourmaline supergroup minerals. Eighteen species have been described to encapsulate the chemical variety found in this intriguing structure. A complexly zoned slice of fl uor-liddi- FIGURE 1 coatite cut parallel to (001), from KEYWORDS: tourmaline, crystal structure, crystal chemistry, pyroelectricity, Anjanabonoina, Madagascar. This slice measures piezoelectricity, tourmaline nomenclature, gemstone approximately 10 cm in its longest dimension, and displays a trigonal star pattern and “aggregate-type” zoning. The outer region consists of fi ne-scale color zoning roughly parallel to the prism faces. INTRODUCTION PHOTO © HAROLD & ERICA VAN PELT For many years, tourmaline sensu lato (i.e. minerals of the CHEMICAL COMPOSITION tourmaline supergroup) was not taken seriously by miner- Our fi rst indication of the complexity of the minerals of alogists, petrologists, and geochemists. -

1 Revision 2 1 Alumino-Oxy-Rossmanite From
1 Revision 2 2 Alumino-oxy-rossmanite from pegmatites in Variscan metamorphic rocks from Eibenstein 3 an der Thaya, Lower Austria, Austria: a new tourmaline that represents the most Al-rich 4 end-member composition 5 1 2 3,4 4 6 ANDREAS ERTL , JOHN M. HUGHES , STEFAN PROWATKE , THOMAS LUDWIG , 1 4 1 5,1 7 CHRISTIAN L. LENGAUER , HANS-PETER MEYER , GERALD GIESTER , UWE KOLITSCH , AND 6 8 ALBERT PRAYER 9 10 1 Institut für Mineralogie und Kristallographie, Geozentrum, Universität Wien, Althanstrasse 14, 11 1090 Wien, Austria 12 2 Department of Geology, University of Vermont, Burlington, VT 05405, U.S.A. 13 3 D. Swarovski KG, Swarovskistrasse 30, 6112 Wattens, Austria 14 4 Institut für Geowissenschaften, Universität Heidelberg, Im Neuenheimer Feld 234–236, 69120 15 Heidelberg, Germany 16 5 Mineralogisch-Petrographische Abt., Naturhistorisches Museum, Burgring 7, 1010 Vienna, 17 Austria 18 6 Sportplatzgasse 8, 3754 Irnfritz, Austria 19 20 21 corresponding author: [email protected] 1 22 ABSTRACT 23 Alumino-oxy-rossmanite, ideally 3Al6(Si5AlO18)(BO3)3(OH)3O, is here described as a new 24 member of the tourmaline supergroup. It is an early-magmatic Al-rich oxy-tourmaline from a 25 small pegmatitic body embedded in amphibolite and biotite-rich paragneiss. This new pink 26 tourmaline was found in a Moldanubian pegmatite (of the Drosendorf Unit) that occurs in a large 27 quarry near the village of Eibenstein an der Thaya, Waidhofen an der Thaya district, Lower 28 Austria, Austria. The empirical formula of the holotype was determined on the basis of electron- 29 microprobe analysis (EMPA), secondary-ion mass spectrometry (SIMS), spectroscopical 30 methods (optical absorption and infrared spectroscopy), and crystal-structure refinement (SREF) X Y 3+ 2+ 3+ 2+ 4+ Z 31 as (0.53Na0.46Ca0.01) (Al2.37Mn 0.21Li0.160.14Mn 0.07Fe 0.03Fe 0.01Ti 0.01) Al6 V W 32 (Si5.37Al0.41B0.22O18)(BO3)3 [(OH)2.77O0.23] [O0.80(OH)0.15F0.05]. -

Povondraite Nafe 6 (BO3)
3+ 3+ Povondraite NaFe3 Fe6 (BO3)3Si6O18(O; OH)4 c 2001 Mineral Data Publishing, version 1.2 ° Crystal Data: Hexagonal. Point Group: 3m: Equant but distorted prisms, terminated by trigonal pyramids, in subparallel aggregates, to several mm. Physical Properties: Fracture: Uneven to conchoidal. Tenacity: Brittle. Hardness = 7 » D(meas.) = 3.26(3) D(calc.) = 3.33 Optical Properties: Nearly opaque. Color: Black. Streak: Brown. Luster: Resinous, more or less splendent. Optical Class: Uniaxial ({). Pleochroism: Strong; O = yellow-brown, dark brown, brown-black; E = dark red-brown, light brown. ! = 1.800{1.820 ² = 1.743{1.751 Cell Data: Space Group: R3m: a = 16.186(2) c = 7.444(1) Z = 3 X-ray Powder Pattern: San Francisco mine, Bolivia. 2.63 (10), 6.63 (9), 4.05 (9), 3.05 (9), 3.61 (8), 5.13 (7), 4.71 (7) Chemistry: (1) SiO2 30.7 TiO2 0.0 B2O3 [9.0] Al2O3 1.4 Fe2O3 45.0 V2O3 trace FeO 1.7 MgO 6.5 K2O 1.0 Na2O 2.1 H2O [2.4] Total [99.8] 2+ 3+ (1) San Francisco mine, Bolivia; by electron microprobe, Fe :Fe and B2O3 calculated from stoichiometry, H2O from charge balance; corresponds to (Na0:80K0:26)§=1:06 3+ 2+ 3+ (Fe2:28Mg0:53Fe0:27)§=3:08(Fe4:29Mg1:36Al0:32)§=5:97(BO3)3Si5:96O18[(OH)3:12O0:99]§=4:00: Mineral Group: Tourmaline group. Occurrence: Very rare, in fractures and lining cavities in schist metamorphosed from sedimentary rocks. Association: Quartz, potassic feldspar, muscovite, schorl, riebeckite, magnesite. Distribution: In the San Francisco mine, near Villa Tunari, Alto Chapare, Cochabamba, Bolivia. -

Tourmalines As a Tool in Provenance Studies of Terrigenous Material in Extra-Carpathian Albian (Uppermost Lower Cretaceous) Sand
minerals Article Tourmalines as a Tool in Provenance Studies of Terrigenous Material in Extra-Carpathian Albian (Uppermost Lower Cretaceous) Sands of Miechów Synclinorium, Southern Poland Jakub Kotowski * , Krzysztof Nejbert and Danuta Olszewska-Nejbert Faculty of Geology, University of Warsaw, Zwirki˙ i Wigury 93, 02-089 Warszawa, Poland; [email protected] (K.N.); [email protected] (D.O.-N.) * Correspondence: [email protected] Received: 31 August 2020; Accepted: 14 October 2020; Published: 16 October 2020 Abstract: The mature transgressive Albian quartz sands in the Miechów Synclinorium contain a poor (<1%) heavy mineral suite consisting of tourmaline, rutile, garnet, staurolite, ilmenite, zircon, monazite, kyanite, and gahnite. The other minerals, especially those containing Fe and Ti (e.g., biotite), are subordinate. Over 512 tourmaline grains from seven outcrops in the Miechów Segment were analysed using electron probe microanalysis (EPMA). The majority of grains belong to the alkali tourmaline group in which the X-site is dominated by Na (0.4 to 0.9 apfu). Detrital tourmalines are mainly dravite with a prevalent schorl end-member with average XMg values over 0.6. Apart from Mg and Fe, the other Y-site cations rarely exceed 0.1 apfu. Fluorine content is usually below the detection limit. Their chemical composition suggests that most tourmaline grains were sourced from Al-rich and Al-poor metapelites/metapsammites. The main source rocks for the Albian sands were rocks from low- to medium-grade metamorphism, probably from Al-rich quartz-muscovite schist and/or muscovite rich gneisses. Additional minor source rocks were granites and pegmatites coexisting with them. -
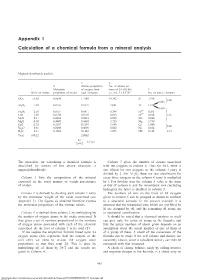
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

Mineralogy and Geochemistry of Tourmaline in Contrasting Hydrothermal Systems: Copiapó Area, Northern Chile
MINERALOGY AND GEOCHEMISTRY OF TOURMALINE IN CONTRASTING HYDROTHERMAL SYSTEMS: COPIAPÓ AREA, NORTHERN CHILE by Ana C. Collins A Prepublication Manuscript Submitted to the Faculty of the DEPARTMENT OF GEOSCIENCES In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 2010 STATEMENT BY THE AUTHOR This thesis has been submitted in partial fulfillment of requirements for the Master of Science degree at the University of Arizona and is deposited in the Antevs Reading Room to be made available to borrowers, as are copies of regular theses and dissertations. Brief quotations from this manuscript are allowable without special permission, provided that accurate acknowledgment of the source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the Department of Geosciences when the proposed use of the material is in the interests of scholarship. In all other instances, however, permission must be obtained by the author. __Ana Collins________________________________ ________________ (author’s signature) (date) APPROVAL BY RESEARCH COMMITTEE As members of the Research Committee, we recommend that this thesis be accepted as fulfilling the research requirement for the degree of Master of Science. _Dr. Mark D. Barton__________________________ ________________ Major Advisor (type name) (signature) (date) _Dr. Eric Seedorff____________________________ ________________ (type name) (signature) (date) _Dr. -

List of Mineral Symbols
THE CANADIAN MINERALOGIST LIST OF SYMBOLS FOR ROCK- AND ORE-FORMING MINERALS (January 1, 2021) ____________________________________________________________________________________________________________ Ac acanthite Ado andorite Asp aspidolite Btr berthierite Act actinolite Adr andradite Ast astrophyllite Brl beryl Ae aegirine Ang angelaite At atokite Bll beryllonite AeAu aegirine-augite Agl anglesite Au gold Brz berzelianite Aen aenigmatite Anh anhydrite Aul augelite Bet betafite Aes aeschynite-(Y) Ani anilite Aug augite Bkh betekhtinite Aik aikinite Ank ankerite Aur auricupride Bdt beudantite Akg akaganeite Ann annite Aus aurostibite Beu beusite Ak åkermanite An anorthite Aut autunite Bch bicchulite Ala alabandite Anr anorthoclase Aw awaruite Bt biotite* Ab albite Atg antigorite Axn axinite-(Mn) Bsm bismite Alg algodonite Sb antimony Azu azurite Bi bismuth All allactite Ath anthophyllite Bdl baddeleyite Bmt bismuthinite Aln allanite Ap apatite* Bns banalsite Bod bohdanowiczite Alo alloclasite Arg aragonite Bbs barbosalite Bhm böhmite Ald alluaudite Ara aramayoite Brr barrerite Bor boralsilite Alm almandine Arf arfvedsonite Brs barroisite Bn bornite Alr almarudite Ard argentodufrénoysite Blt barylite Bou boulangerite Als alstonite Apn argentopentlandite Bsl barysilite Bnn bournonite Alt altaite Arp argentopyrite Brt baryte, barite Bow bowieite Aln alunite Agt argutite Bcl barytocalcite Brg braggite Alu alunogen Agy argyrodite Bss bassanite Brn brannerite Amb amblygonite Arm armangite Bsn bastnäsite Bra brannockite Ams amesite As arsenic