2000: 213–235
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Revision and Phylogeny of Acalypha (Euphorbiaceae) in Malesia
Blumea 55, 2010: 21–60 www.ingentaconnect.com/content/nhn/blumea RESEARCH ARTICLE doi:10.3767/000651910X499141 Revision and phylogeny of Acalypha (Euphorbiaceae) in Malesia V.G. Sagun1,2, G.A. Levin2, P.C. van Welzen3 Key words Abstract Twenty-eight species of Acalypha are recognized in Malesia. Acalypha paniculata is the sole member of subgenus Linostachys in Malesia and the rest of the species belong to subgenus Acalypha. Four previously Acalypha synonymized species are resurrected as distinct species, namely A. angatensis, A. cardiophylla var. cardiophylla, Euphorbiaceae A. grandis, and A. wilkesiana. Four species names are newly reduced to synonymy. The molecular phylogenetic Malesia analyses indicate that Acalypha is monophyletic, as is the subgenus Acalypha. The early-diverging lineages in the phylogeny genus, and its closest outgroup, consist of African species. The Malesian species do not form a monophyletic group although the molecular data strongly support two small clades within the region that are morphologically homogene- ous. The classification system that Pax and Hoffmann applied to subgenus Acalypha, which is based primarily on inflorescence morphology, appears to be unsatisfactory and incongruent with the phylogenetic analyses. Published on 16 April 2010 INTRODUCTION Molecular systematics confirms the placement of Acalypha in Acalyphoideae s.s. and shows a close relationship between Acalypha L. is the third largest genus in the Euphorbiaceae Acalypha and Mareya Baill. (Wurdack et al. 2005, Tokuoka s.s. after Euphorbia L., and Croton L., having about 450 spe- 2007). Their relationship is supported by similar morphologi- cies worldwide (Webster 1994, Radcliffe-Smith 2001). In the cal characteristics, including laciniate styles, pendulous anther Malesian region, 28 species of Acalypha are recognized herein. -

CHAPTER 2 REVIEW of the LITERATURE 2.1 Taxa And
CHAPTER 2 REVIEW OF THE LITERATURE 2.1 Taxa and Classification of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.11 Taxa and Classification of Acalypha indica Linn. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Euphorbiales Family : Euphorbiaceae Subfamily : Acalyphoideae Genus : Acalypha Species : Acalypha indica Linn. (Saha and Ahmed, 2011) Plant Synonyms: Acalypha ciliata Wall., A. canescens Wall., A. spicata Forsk. (35) Common names: Brennkraut (German), alcalifa (Brazil) and Ricinela (Spanish) (36). 9 2.12 Taxa and Classification of Bridelia retusa (L.) A. Juss. Kingdom : Plantae Division : Magnoliophyta Class : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Bridelia Species : Bridelia retusa (L.) A. Juss. Plant Synonyms: Bridelia airy-shawii Li. Common names: Ekdania (37,38). 2.13 Taxa and Classification of Cleidion javanicum BL. Kingdom : Plantae Subkingdom : Tracheobionta Superdivision : Spermatophyta Division : Magnoliophyta Class : Magnoliopsida Subclass : Magnoliopsida Order : Malpighiales Family : Euphorbiaceae Genus : Cleidion Species : Cleidion javanicum BL. Plant Synonyms: Acalypha spiciflora Burm. f. , Lasiostylis salicifolia Presl. Cleidion spiciflorum (Burm.f.) Merr. Common names: Malayalam and Yellari (39). 10 2.2 Review of chemical composition and bioactivities of Acalypha indica Linn., Bridelia retusa (L.) A. Juss. and Cleidion javanicum BL. 2.2.1 Review of chemical composition and bioactivities of Acalypha indica Linn. Acalypha indica -
![Entry for ACALYPHA Acrogyna Pax [Family EUPHORBIACEAE]](https://docslib.b-cdn.net/cover/8673/entry-for-acalypha-acrogyna-pax-family-euphorbiaceae-78673.webp)
Entry for ACALYPHA Acrogyna Pax [Family EUPHORBIACEAE]
Entry for ACALYPHA acrogyna Pax [family EUPHORBIACEAE] http://plants.jstor.org/flora/flota011327 http://www.jstor.org Your use of the JSTOR archive indicates your acceptance of JSTOR's Terms and Conditions of Use, available at http://www.jstor.org/page/info/about/policies/terms.jsp. JSTOR's Terms and Conditions of Use provides, in part, that unless you have obtained prior permission, you may not download an entire issue of a journal or multiple copies of articles, and you may use content in the JSTOR archive only for your personal, non-commercial use. Please contact the contributing partner regarding any further use of this work. Partner contact information may be obtained at http://plants.jstor.org/page/about/plants/PlantsProject.jsp. Each copy of any part of a JSTOR transmission must contain the same copyright notice that appears on the screen or printed page of such transmission. JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Page 1 of 2 Entry for ACALYPHA acrogyna Pax [family EUPHORBIACEAE] Herbarium Royal Botanic Gardens, Kew (K) Collection Flora of Tropical Africa Resource Type Reference Sources Entry from Flora of Tropical Africa, Vol 6 Part 1, page 441 (1913) Author: (By J. G. Baker, with additions by C. H. Wright.) Names ACALYPHA acrogyna Pax [family EUPHORBIACEAE], in Engl. Jahrb. -
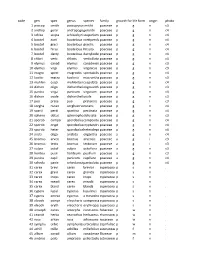
Species List (PDF)
code gen spec genus species family growth formlife form origin photo 1 pascop smith pascopyrumsmithii poaceae p g n c3 2 androp gerar andropogongerardii poaceae p g n c4 3 schiza scopa schizachyriumscoparium poaceae p g n c4 4 boutel curti bouteloua curtipendulapoaceae p g n c4 5 boutel graci bouteloua gracilis poaceae p g n c4 6 boutel hirsu bouteloua hirsuta poaceae p g n c4 7 boutel dacty bouteloua dactyloidespoaceae p g n c4 8 chlori verti chloris verticillata poaceae p g n c4 9 elymus canad elymus canadensispoaceae p g n c3 10 elymus virgi elymus virginicus poaceae p g n c3 11 eragro spect eragrostis spectabilis poaceae p g n c4 12 koeler macra koeleria macrantha poaceae p g n c3 13 muhlen cuspi muhlenbergiacuspidata poaceae p g n c4 14 dichan oligo dichantheliumoligosanthespoaceae p g n c3 15 panicu virga panicum virgatum poaceae p g n c4 16 dichan ovale dichantheliumovale poaceae p g n c3 17 poa prate poa pratensis poaceae p g i c3 18 sorgha nutan sorghastrumnutans poaceae p g n c4 19 sparti pecti spartina pectinata poaceae p g n c4 20 spheno obtus sphenopholisobtusata poaceae p g n c3 21 sporob compo sporoboluscomposituspoaceae p g n c4 22 sporob crypt sporoboluscryptandruspoaceae p g n c4 23 sporob heter sporobolusheterolepispoaceae p g n c4 24 aristi oliga aristida oligantha poaceae a g n c4 25 bromus arven bromus arvensis poaceae a g i c3 26 bromus tecto bromus tectorum poaceae a g i c3 27 vulpia octof vulpia octoflora poaceae a g n c3 28 hordeu pusil hordeum pusillum poaceae a g n c3 29 panicu capil panicum capillare poaceae a g n c4 30 schedo panic schedonnarduspaniculatuspoaceae p g n c4 31 carex brevi carex brevior cyperaceaep s n . -

Apiales, Aquifoliales, Boraginales, , Brassicales, Canellales
Kingdom: Plantae Phylum: Tracheophyta Class: Magnoliopsida Order: Apiales, Aquifoliales, Boraginales, , Brassicales, Canellales, Caryophyllales, Celastrales, Ericales, Fabales, Garryales, Gentianales, Lamiales, Laurales, Magnoliales, Malpighiales, Malvales, Myrtales, Oxalidales, Picramniales, Piperales, Proteales, Rosales, Santalales, Sapindales, Solanales Family: Achariaceae, Anacardiaceae, Annonaceae, Apocynaceae, Aquifoliaceae, Araliaceae, Bignoniaceae, Bixaceae, Boraginaceae, Burseraceae, Calophyllaceae, Canellaceae, Cannabaceae, Capparaceae, Cardiopteridaceae, Caricaceae, Caryocaraceae, Celastraceae, Chrysobalanaceae, Clusiaceae, Combretaceae, Dichapetalaceae, Ebenaceae, Elaeocarpaceae, Emmotaceae, Erythroxylaceae, Euphorbiaceae, Fabaceae, Goupiaceae, Hernandiaceae, Humiriaceae, Hypericaceae, Icacinaceae, Ixonanthaceae, Lacistemataceae, Lamiaceae, Lauraceae, Lecythidaceae, Lepidobotryaceae, Linaceae, Loganiaceae, Lythraceae, Malpighiaceae, Malvaceae, Melastomataceae, Meliaceae, Monimiaceae, Moraceae, Myristicaceae, Myrtaceae, Nyctaginaceae, Ochnaceae, Olacaceae, Oleaceae, Opiliaceae, Pentaphylacaceae, Phyllanthaceae, Picramniaceae, Piperaceae, Polygonaceae, Primulaceae, Proteaceae, Putranjivaceae, Rhabdodendraceae, Rhamnaceae, Rhizophoraceae, Rosaceae, Rubiaceae, Rutaceae, Sabiaceae, Salicaceae, Sapindaceae, Sapotaceae, Simaroubaceae, Siparunaceae, Solanaceae, Stemonuraceae, Styracaceae, Symplocaceae, Ulmaceae, Urticaceae, Verbenaceae, Violaceae, Vochysiaceae Genus: Abarema, Acioa, Acosmium, Agonandra, Aiouea, Albizia, Alchornea, -

Czech University of Life Sciences Prague
Czech University of Life Sciences Prague Faculty of Tropical AgriSciences Molecular Characterization of Plukenetia volubilis L. and Analysis of Seed Storage Protein Pattern and Protein Fractions Dissertation Thesis Department of Crop Sciences and Agroforestry Author: Ing. Martin Ocelák Supervisor: doc. Ing. Bohdan Lojka, Ph.D. Co-supervisors: Ing. Petra Hlásná Čepková, Ph.D. Ing. Iva Viehmannová, Ph.D. In Prague, September, 2016 Acknowledgment I would like to express my gratitude to my supervisor doc. Ing. Bohdan Lojka, Ph.D. and co-supervisors Ing. Petra Hlásná Čepková, Ph.D. and Ing. Iva Viehmannová, Ph.D. for their guidance, advices, help and also patience during the studies, laboratory works and mainly during the writings. My thanks also belong to IIAP represented by Ing. Danter Cachique Huansi and Lucas Garcia Chujutalli for their cooperation in samples collection, to Ing. Anna Prohasková for her guidance during analysis of proteins in Crop Research Institute in Prague - Ruzyně, to Ing. Eva Beoni, Ph.D. and Ing. Lenka Havlíčková, Ph.D. for their help in learning how to work in the laboratory; to Ing. Zdislava Dvořáková, Ph.D. for her help, teaching and encouragement and to Ing. Blanka Křivánková, Ph.D. for providing some useful materials. Also my family contributed with their support in all means. So great thanks belong to my parents Jan and Jaroslava Ocelákovi and my boyfriend Ioannis Nikolakis for their love and support in all possible means. This research was supported financially by an Internal Grant Agency of the University of Life Science Prague, CIGA (Project No. 20135004), by an Internal Grant Agency of the Faculty of Tropical AgriSciences - University of Life Science Prague, IGA (Project No. -

Patrones De Endemismo Y Disyunción De Los Géneros De Euphorbiaceae Sensu Lato: Un Análisis Panbiogeográfico
Boletín de la Sociedad Botánica de México 77: 21-33, 2005 DOI: 10.17129/botsci.1710 Bol.Soc.Bot.Méx. 77: 21-33 (2005) SISTEMÁTICA Y FLORÍSTICA PATRONES DE ENDEMISMO Y DISYUNCIÓN DE LOS GÉNEROS DE EUPHORBIACEAE SENSU LATO: UN ANÁLISIS PANBIOGEOGRÁFICO MARTHA MARTÍNEZ-GORDILLO1 Y JUAN J. MORRONE2 1 Herbario “FCME”, Departamento de Biología Comparada, Facultad de Ciencias, Universidad Nacional Autónoma de México. Apdo. Postal 70-181, México 04510, D.F., México. Correo-e: [email protected]. 2 Museo de Zoología “Alfonso L. Herrera”, Departamento de Biología Evolutiva, Facultad de Ciencias, Universidad Nacional Autónoma de México. Apdo. Postal 70-399, México 04510, D.F., México Correo-e: [email protected]. Resumen: Se analizaron los patrones de distribución de los géneros de Euphorbiaceae bajo un enfoque panbiogeográfico, emplean- do el método del análisis de parsimonia de endemismos (PAE). Se obtuvieron cuatro trazos generalizados, que unen las regiones siguientes: (1) Neotropical-Afrotropical (determinado por los géneros Amanoa, Caperonia, Conceveiba, Manprounea, Pogonophora, Savia y Tetrorchidium); (2) Australiana Templada-Australiana Tropical-Neoguineana-Oriental (determinado por los géneros Actephila, Baloghia, Choriceras, Petalostigma y Sauropus); (3) Australiana Templada-Australiana Tropical- Neoguineana-Afrotropical-Neotropical (determinado por los géneros Acalypha, Alchornea, Cleidion, Drypetes, Margaritaria, Microstachys, Omphalea y Phyllanthus); y (4) Neoguineana-Oriental-Afrotopical (determinado por los géneros Glochidion, Macaranga, Microdesmis y Shirakopsis). Dos trazos generalizados se superponen en la región Afrotropical, la cual es identifica- da como un nodo. Palabras clave: biogeografía, distribución, endemismo, Euphorbiaceae s. l.,PAE, trazos generalizados. Abstract: Distributional patterns of the genera of Euphorbiaceae were analyzed under a panbiogeographic approach, using the parsimony analysis of endemicity (PAE) method. -

Revision of the Genus Cleidion (Euphorbiaceae) in Malesia
BLUMEA 50: 197–219 Published on 22 April 2005 http://dx.doi.org/10.3767/000651905X623373 REVISION OF THE GENUS CLEIDION (EUPHORBIACEAE) IN MALESIA KRISTO K.M. KULJU & PETER C. VAN WELZEN Nationaal Herbarium Nederland, Universiteit Leiden branch, P.O. Box 9514, 2300 RA Leiden, The Netherlands; e-mail: [email protected], [email protected] SUMMARY A revision of the Malesian species in the genus Cleidion is presented. Cleidion javanicum is shown to be the correct name for the widespread type species (instead of the name C. spiciflorum). A new species, C. luziae, resembling C. javanicum, is described from the Moluccas, New Guinea and the Solomon Islands. In addition, C. salomonis is synonymised with C. papuanum and C. lanceolatum is treated as a variety of C. ramosii. In total 7 Malesian Cleidion species are recognized. Cleidion megistophyllum from the Philippines cannot reliably be confirmed to belong to the genus due to lack of information and specimens and is treated as a doubtful species. Key words: Cleidion, Acalypheae, Cleidiinae, revision, taxonomy, Malesia. INTRODUCTION Cleidion is a pantropical genus belonging to the large angiosperm family Euphorbiaceae s.s. It was described by Blume (1825), who included a single species C. javanicum1. The first revision was made by Müller Argoviensis (1865, 1866). His work was fol- lowed by the comprehensive treatment of Pax & Hoffmann (1914), which included 17 species. Pax & Hoffmann excluded the section Discocleidion Müll.Arg. which differs from Cleidion by the presence of a staminate and pistillate disc (in Cleidion a disc is absent), stipellate and palmatinerved leaves (in Cleidion the leaves are non-stipellate and pinnatinerved), and differences in anther type. -

Annals of the Missouri Botanical Garden 1988
- Annals v,is(i- of the Missouri Botanical Garden 1988 # Volume 75 Number 1 Volume 75, Number ' Spring 1988 The Annals, published quarterly, contains papers, primarily in systematic botany, con- tributed from the Missouri Botanical Garden, St. Louis. Papers originating outside the Garden will also be accepted. Authors should write the Editor for information concerning arrangements for publishing in the ANNALS. Instructions to Authors are printed on the inside back cover of the last issue of each volume. Editorial Committee George K. Rogers Marshall R. Crosby Editor, Missouri B Missouri Botanical Garden Editorial is. \I,,S ouri Botanu •al Garde,, John I). Dwyer Missouri Botanical Garden Saint Louis ( niversity Petei • Goldblatt A/I.S.S ouri Botanic al Garder Henl : van der W< ?rff V//.S.S ouri Botanic tor subscription information contact Department IV A\NM.S OK Tin: Missot m Boi >LM« M G\KDE> Eleven, P.O. Box 299, St. Louis, MO 63166. Sub- (ISSN 0026-6493) is published quarterly by the scription price is $75 per volume U.S., $80 Canada Missouri Botanical Garden, 2345 Tower Grove Av- and Mexico, $90 all other countries. Airmail deliv- enue, St. Louis, MO 63110. Second class postage ery charge, $35 per volume. Four issues per vol- paid at St. Louis, MO and additional mailing offices. POSTMAS'IKK: Send ad«lrt— changes to Department i Botanical Garden 1988 REVISED SYNOPSIS Grady L. Webster2 and Michael J. Huft" OF PANAMANIAN EUPHORBIACEAE1 ABSTRACT species induded in \ • >,H The new taxa ai I. i i " I ! I _- i II • hster, Tragia correi //,-," |1 U !. -

Redalyc.STRUCTURE of the SHRUB-ARBOREAL COMPONENT
Interciencia ISSN: 0378-1844 [email protected] Asociación Interciencia Venezuela Gabriel Christo, Alexandre; Guedes-Bruni, Rejan R.; Araújo P. Sobrinho, Felipe de; Gomes da Silva, Ary; Luna Peixoto, Ariane STRUCTURE OF THE SHRUB-ARBOREAL COMPONENT OF AN ATLANTIC FOREST FRAGMENT ON A HILLOCK IN THE CENTRAL LOWLAND OF RIO DE JANEIRO, BRAZIL Interciencia, vol. 34, núm. 4, abril, 2009, pp. 232-239 Asociación Interciencia Caracas, Venezuela Available in: http://www.redalyc.org/articulo.oa?id=33911575002 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative STRUCTURE OF THE SHRUB-ARBOREAL COMPONENT OF AN ATLANTIC FOREST FRAGMENT ON A HILLOCK IN THE CENTRAL LOWLAND OF RIO DE JANEIRO, BRAZIL AleXandre Gabriel Christo, Rejan R. Guedes-Bruni, Felipe DE AraÚjo P. Sobrinho, Ary Gomes DA SilVA and Ariane Luna PeiXoto SUMMARY The present study describes and evaluates the horizontal and ver- species with the greatest importance values (VI) were Aparisthmium tical structures of a lowland forest fragment on a hillock in the mu- cordatum, Guapira opposita, Lacistema pubescens, Xylopia sericea, nicipality of Silva Jardim, Rio de Janeiro State, Brazil (22o31’56’’S Tapirira guianensis and Piptocarpha macropoda. The high diversity and 42o20’46’’W). Twenty plots (10×2m) totaling 0.5ha were laid out observed was influenced by earlier anthropogenic actions and by the following the slope grade using DBH≥5cm as the inclusion criterion. current successional stage. The forest fragment studied demonstrated A total of 734 individuals were encountered, yielding a total density closer floristic similarity to areas inventoried in a close-by biologi- of 1468 ind./ha and a total basal area of 10783m2. -

Chec List What Survived from the PLANAFLORO Project
Check List 10(1): 33–45, 2014 © 2014 Check List and Authors Chec List ISSN 1809-127X (available at www.checklist.org.br) Journal of species lists and distribution What survived from the PLANAFLORO Project: PECIES S Angiosperms of Rondônia State, Brazil OF 1* 2 ISTS L Samuel1 UniCarleialversity of Konstanz, and Narcísio Department C.of Biology, Bigio M842, PLZ 78457, Konstanz, Germany. [email protected] 2 Universidade Federal de Rondônia, Campus José Ribeiro Filho, BR 364, Km 9.5, CEP 76801-059. Porto Velho, RO, Brasil. * Corresponding author. E-mail: Abstract: The Rondônia Natural Resources Management Project (PLANAFLORO) was a strategic program developed in partnership between the Brazilian Government and The World Bank in 1992, with the purpose of stimulating the sustainable development and protection of the Amazon in the state of Rondônia. More than a decade after the PLANAFORO program concluded, the aim of the present work is to recover and share the information from the long-abandoned plant collections made during the project’s ecological-economic zoning phase. Most of the material analyzed was sterile, but the fertile voucher specimens recovered are listed here. The material examined represents 378 species in 234 genera and 76 families of angiosperms. Some 8 genera, 68 species, 3 subspecies and 1 variety are new records for Rondônia State. It is our intention that this information will stimulate future studies and contribute to a better understanding and more effective conservation of the plant diversity in the southwestern Amazon of Brazil. Introduction The PLANAFLORO Project funded botanical expeditions In early 1990, Brazilian Amazon was facing remarkably in different areas of the state to inventory arboreal plants high rates of forest conversion (Laurance et al. -

Title Evolutionary Relationships Between Pollination and Protective Mutualisms in the Genus Macaranga (Euphorbiaceae)( Dissertat
Evolutionary relationships between pollination and protective Title mutualisms in the genus Macaranga (Euphorbiaceae)( Dissertation_全文 ) Author(s) Yamasaki, Eri Citation 京都大学 Issue Date 2014-03-24 URL https://doi.org/10.14989/doctor.k18113 学位規則第9条第2項により要約公開; 許諾条件により本文 Right は2019-06-25に公開 Type Thesis or Dissertation Textversion ETD Kyoto University Evolutionary relationships between pollination and protective mutualisms in the genus Macaranga (Euphorbiaceae) Eri Yamasaki 2014 1 2 Contents 摘要.…………………………………………………………………………………..5 Summary.……………………………………………………………………………..9 Chapter 1 General introduction……………………………………………………………….14 Chapter 2 Diversity of pollination systems in Macaranga Section 2.1 Diversity of bracteole morphology in Macaranga ………………………….20 Section 2.2 Wind and insect pollination (ambophily) in Mallotus , a sister group of Macaranga …………..…………..……...…………..………………………...31 Section 2.3 Disk-shaped nectaries on bracteoles of Macaranga sinensis provide a reward for pollinators……………………………….………………………………...45 Chapter 3 Interactions among plants, pollinators and guard ants in ant-plant Macaranga Section 3.1 Density of ant guards on inflorescences and their effects on herbivores and pollinators…………………………………………………….......................56 Section 3.2 Anal secretions of pollinator thrips of Macaranga winkleri repel guard ants…….71 Chapter 4 General discussion.………………….……………………………………………...85 Appendix…………………………………………………………………….………89 Acknowledgement…………………………………………………………….…...101 Literature cited……………………………….…………………………………….103