Cryopreservation of Cyanobacteria and Eukaryotic Microalgae Using Exopolysaccharide Extracted from a Glacier Bacterium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide
antioxidants Review Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide Ivan Kushkevych 1,* , Veronika Bosáková 1,2 , Monika Vítˇezová 1 and Simon K.-M. R. Rittmann 3,* 1 Department of Experimental Biology, Faculty of Science, Masaryk University, 62500 Brno, Czech Republic; [email protected] (V.B.); [email protected] (M.V.) 2 Department of Biology, Faculty of Medicine, Masaryk University, 62500 Brno, Czech Republic 3 Archaea Physiology & Biotechnology Group, Department of Functional and Evolutionary Ecology, Universität Wien, 1090 Vienna, Austria * Correspondence: [email protected] (I.K.); [email protected] (S.K.-M.R.R.); Tel.: +420-549-495-315 (I.K.); +431-427-776-513 (S.K.-M.R.R.) Abstract: Hydrogen sulfide is a toxic compound that can affect various groups of water microorgan- isms. Photolithotrophic sulfur bacteria including Chromatiaceae and Chlorobiaceae are able to convert inorganic substrate (hydrogen sulfide and carbon dioxide) into organic matter deriving energy from photosynthesis. This process takes place in the absence of molecular oxygen and is referred to as anoxygenic photosynthesis, in which exogenous electron donors are needed. These donors may be reduced sulfur compounds such as hydrogen sulfide. This paper deals with the description of this metabolic process, representatives of the above-mentioned families, and discusses the possibility using anoxygenic phototrophic microorganisms for the detoxification of toxic hydrogen sulfide. Moreover, their general characteristics, morphology, metabolism, and taxonomy are described as Citation: Kushkevych, I.; Bosáková, well as the conditions for isolation and cultivation of these microorganisms will be presented. V.; Vítˇezová,M.; Rittmann, S.K.-M.R. -

Limits of Life on Earth Some Archaea and Bacteria
Limits of life on Earth Thermophiles Temperatures up to ~55C are common, but T > 55C is Some archaea and bacteria (extremophiles) can live in associated usually with geothermal features (hot springs, environments that we would consider inhospitable to volcanic activity etc) life (heat, cold, acidity, high pressure etc) Thermophiles are organisms that can successfully live Distinguish between growth and survival: many organisms can survive intervals of harsh conditions but could not at high temperatures live permanently in such conditions (e.g. seeds, spores) Best studied extremophiles: may be relevant to the Interest: origin of life. Very hot environments tolerable for life do not seem to exist elsewhere in the Solar System • analogs for extraterrestrial environments • `extreme’ conditions may have been more common on the early Earth - origin of life? • some unusual environments (e.g. subterranean) are very widespread Extraterrestrial Life: Spring 2008 Extraterrestrial Life: Spring 2008 Grand Prismatic Spring, Yellowstone National Park Hydrothermal vents: high pressure in the deep ocean allows liquid water Colors on the edge of the at T >> 100C spring are caused by different colonies of thermophilic Vents emit superheated water (300C or cyanobacteria and algae more) that is rich in minerals Hottest water is lifeless, but `cooler’ ~50 species of such thermophiles - mostly archae with some margins support array of thermophiles: cyanobacteria and anaerobic photosynthetic bacteria oxidize sulphur, manganese, grow on methane + carbon monoxide etc… Sulfolobus: optimum T ~ 80C, minimum 60C, maximum 90C, also prefer a moderately acidic pH. Live by oxidizing sulfur Known examples can grow (i.e. multiply) at temperatures which is abundant near hot springs. -

PROTISTS Shore and the Waves Are Large, Often the Largest of a Storm Event, and with a Long Period
(seas), and these waves can mobilize boulders. During this phase of the storm the rapid changes in current direction caused by these large, short-period waves generate high accelerative forces, and it is these forces that ultimately can move even large boulders. Traditionally, most rocky-intertidal ecological stud- ies have been conducted on rocky platforms where the substrate is composed of stable basement rock. Projec- tiles tend to be uncommon in these types of habitats, and damage from projectiles is usually light. Perhaps for this reason the role of projectiles in intertidal ecology has received little attention. Boulder-fi eld intertidal zones are as common as, if not more common than, rock plat- forms. In boulder fi elds, projectiles are abundant, and the evidence of damage due to projectiles is obvious. Here projectiles may be one of the most important defi ning physical forces in the habitat. SEE ALSO THE FOLLOWING ARTICLES Geology, Coastal / Habitat Alteration / Hydrodynamic Forces / Wave Exposure FURTHER READING Carstens. T. 1968. Wave forces on boundaries and submerged bodies. Sarsia FIGURE 6 The intertidal zone on the north side of Cape Blanco, 34: 37–60. Oregon. The large, smooth boulders are made of serpentine, while Dayton, P. K. 1971. Competition, disturbance, and community organi- the surrounding rock from which the intertidal platform is formed zation: the provision and subsequent utilization of space in a rocky is sandstone. The smooth boulders are from a source outside the intertidal community. Ecological Monographs 45: 137–159. intertidal zone and were carried into the intertidal zone by waves. Levin, S. A., and R. -

Algae and Cyanobacteria in Coastal and Estuarine Waters
CHAPTER 7 Algae and cyanobacteria in coastal and estuarine waters n coastal and estuarine waters, algae range from single-celled forms to the seaweeds. ICyanobacteria are organisms with some characteristics of bacteria and some of algae. They are similar in size to the unicellular algae and, unlike other bacteria, contain blue-green or green pigments and are able to perform photosynthesis; thus, they are also termed blue-green algae. Algal blooms in the sea have occurred throughout recorded history but have been increasing during recent decades (Anderson, 1989; Smayda, 1989a; Hallegraeff, 1993). In several areas (e.g., the Baltic and North seas, the Adriatic Sea, Japanese coastal waters and the Gulf of Mexico), algal blooms are a recurring phe- nomenon. The increased frequency of occurrence has accompanied nutrient enrich- ment of coastal waters on a global scale (Smayda, 1989b). Blooms of non-toxic phytoplankton species and mass occurrences of macro-algae can affect the amenity value of recreational waters due to reduced transparency, dis- coloured water and scum formation. Furthermore, bloom degradation can be accom- panied by unpleasant odours, resulting in aesthetic problems (see chapter 9). Several human diseases have been reported to be associated with many toxic species of dinoflagellates, diatoms, nanoflagellates and cyanobacteria that occur in the marine environment (CDC, 1997). The effects of these algae on humans are due to some of their constituents, principally algal toxins. Marine algal toxins become a problem primarily because they may concentrate in shellfish and fish that are subsequently eaten by humans (CDR, 1991; Lehane, 2000), causing syndromes known as para- lytic shellfish poisoning (PSP), diarrhetic shellfish poisoning (DSP), amnesic shellfish poisoning (ASP), neurotoxic shellfish poisoning (NSP) and ciguatera fish poisoning (CFP). -

Selection of Cryoprotectant in Lyophilization of Progesterone-Loaded Stearic Acid Solid Lipid Nanoparticles
pharmaceutics Article Selection of Cryoprotectant in Lyophilization of Progesterone-Loaded Stearic Acid Solid Lipid Nanoparticles Timothy M. Amis, Jwala Renukuntla, Pradeep Kumar Bolla and Bradley A. Clark * Department of Basic Pharmaceutical Sciences, Fred Wilson School of Pharmacy, High Point University, High Point, NC 27268, USA; [email protected] (T.M.A.); [email protected] (J.R.); [email protected] (P.K.B.) * Correspondence: [email protected]; Tel.: +1-336-841-9665 Received: 18 August 2020; Accepted: 16 September 2020; Published: 19 September 2020 Abstract: Cryoprotectants are often required in lyophilization to reduce or eliminate agglomeration of solute or suspended materials. The aim of this study was to select a cryoprotecting agent and optimize its concentration in a solid lipid nanoparticle formulation. Progesterone-loaded stearic acid solid lipid nanoparticles (SA-P SLNs) were prepared by hot homogenization with high speed mixing and sonication. The stearic acid content was 4.6% w/w and progesterone was 0.46% w/w of the initial formulation. Multiple surfactants were evaluated, and a lecithin and sodium taurocholate system was chosen. Three concentrations of surfactant were then evaluated, and a concentration of 2% w/w was chosen based on particle size, polydispersity, and zeta potential. Agglomeration of SA-P SLNs after lyophilization was observed as measured by increased particle size. Dextran, glycine, mannitol, polyvinylpyrrolidone (PVP), sorbitol, and trehalose were evaluated as cryoprotectants by both an initial freeze–thaw analysis and after lyophilization. Once selected as the cryoprotectant, trehalose was evaluated at 5%, 10%, 15%, and 20% for optimal concentration, with 20% trehalose being finally selected as the level of choice. -
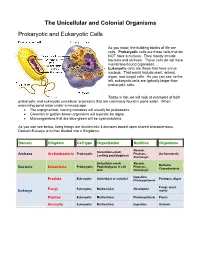
The Unicellular and Colonial Organisms Prokaryotic And
The Unicellular and Colonial Organisms Prokaryotic and Eukaryotic Cells As you know, the building blocks of life are cells. Prokaryotic cells are those cells that do NOT have a nucleus. They mostly include bacteria and archaea. These cells do not have membrane-bound organelles. Eukaryotic cells are those that have a true nucleus. That would include plant, animal, algae, and fungal cells. As you can see, to the left, eukaryotic cells are typically larger than prokaryotic cells. Today in lab, we will look at examples of both prokaryotic and eukaryotic unicellular organisms that are commonly found in pond water. When examining pond water under a microscope… The unpigmented, moving microbes will usually be protozoans. Greenish or golden-brown organisms will typically be algae. Microorganisms that are blue-green will be cyanobacteria. As you can see below, living things are divided into 3 domains based upon shared characteristics. Domain Eukarya is further divided into 4 Kingdoms. Domain Kingdom Cell type Organization Nutrition Organisms Absorb, Unicellular-small; Prokaryotic Photsyn., Archaeacteria Archaea Archaebacteria Lacking peptidoglycan Chemosyn. Unicellular-small; Absorb, Bacteria, Prokaryotic Peptidoglycan in cell Photsyn., Bacteria Eubacteria Cyanobacteria wall Chemosyn. Ingestion, Eukaryotic Unicellular or colonial Protozoa, Algae Protista Photosynthesis Fungi, yeast, Fungi Eukaryotic Multicellular Absorption Eukarya molds Plantae Eukaryotic Multicellular Photosynthesis Plants Animalia Eukaryotic Multicellular Ingestion Animals Prokaryotic Organisms – the archaea, non-photosynthetic bacteria, and cyanobacteria Archaea - Microorganisms that resemble bacteria, but are different from them in certain aspects. Archaea cell walls do not include the macromolecule peptidoglycan, which is always found in the cell walls of bacteria. Archaea usually live in extreme, often very hot or salty environments, such as hot mineral springs or deep-sea hydrothermal vents. -

Archaeal Distribution and Abundance in Water Masses of the Arctic Ocean, Pacific Sector
Vol. 69: 101–112, 2013 AQUATIC MICROBIAL ECOLOGY Published online April 30 doi: 10.3354/ame01624 Aquat Microb Ecol FREEREE ACCESSCCESS Archaeal distribution and abundance in water masses of the Arctic Ocean, Pacific sector Chie Amano-Sato1, Shohei Akiyama1, Masao Uchida2, Koji Shimada3, Motoo Utsumi1,* 1University of Tsukuba, Tennodai, Tsukuba, Ibaraki 305-8572, Japan 2National Institute for Environmental Studies, Onogawa, Tsukuba, Ibaraki 305-8506, Japan 3Tokyo University of Marine Science and Technology, Konan, Minato-ku, Tokyo 108-8477, Japan ABSTRACT: Marine planktonic Archaea have been recently recognized as an ecologically impor- tant component of marine prokaryotic biomass in the world’s oceans. Their abundance and meta- bolism are closely connected with marine geochemical cycling. We evaluated the distribution of planktonic Archaea in the Pacific sector of the Arctic Ocean using fluorescence in situ hybridiza- tion (FISH) with catalyzed reporter deposition (CARD-FISH) and performed statistical analyses using data for archaeal abundance and geochemical variables. The relative abundance of Thaum - archaeota generally increased with depth, and euryarchaeal abundance was the lowest of all planktonic prokaryotes. Multiple regression analysis showed that the thaumarchaeal relative abundance was negatively correlated with ammonium and dissolved oxygen concentrations and chlorophyll fluorescence. Canonical correspondence analysis showed that archaeal distributions differed with oceanographic water masses; in particular, Thaumarchaeota were abundant from the halocline layer to deep water, where salinity was higher and most nutrients were depleted. However, at several stations on the East Siberian Sea side of the study area and along the North- wind Ridge, Thaumarchaeota and Bacteria were proportionally very abundant at the bottom in association with higher nutrient conditions. -

About Cyanobacteria BACKGROUND Cyanobacteria Are Single-Celled Organisms That Live in Fresh, Brackish, and Marine Water
About Cyanobacteria BACKGROUND Cyanobacteria are single-celled organisms that live in fresh, brackish, and marine water. They use sunlight to make their own food. In warm, nutrient-rich environments, microscopic cyanobacteria can grow quickly, creating blooms that spread across the water’s surface and may become visible. Because of the color, texture, and location of these blooms, the common name for cyanobacteria is blue-green algae. However, cyanobacteria are related more closely to bacteria than to algae. Cyanobacteria are found worldwide, from Brazil to China, Australia to the United States. In warmer climates, these organisms can grow year-round. Scientists have called cyanobacteria the origin of plants, and have credited cyanobacteria with providing nitrogen fertilizer for rice and beans. But blooms of cyanobacteria are not always helpful. When these blooms become harmful to the environment, animals, and humans, scientists call them cyanobacterial harmful algal blooms (CyanoHABs). Freshwater CyanoHABs can use up the oxygen and block the sunlight that other organisms need to live. They also can produce powerful toxins that affect the brain and liver of animals and humans. Because of concerns about CyanoHABs, which can grow in drinking water and recreational water, the U.S. Environmental Protection Agency (EPA) has added cyanobacteria to its Drinking Water Contaminant Candidate List. This list identifies organisms and toxins that EPA considers to be priorities for investigation. ASSESSING THE IMPACT ON PUBLIC HEALTH Reports of poisonings associated with CyanoHABs date back to the late 1800s. Anecdotal evidence and data from laboratory animal research suggest that cyanobacterial toxins can cause a range of adverse human health effects, yet few studies have explored the links between CyanoHABs and human health. -

Cryoprotectant Production Capacity of the Freeze-Tolerant Wood Frog, Rana Sylvatica
71 Cryoprotectant production capacity of the freeze-tolerant wood frog, Rana sylvatica JoN P. Cosr,cNzo AND Rlcnano E. LsE, Jn. Department of Zoology, Miami University, Oxford, OH 45056, U.S.A. ReceivedJune 8. 1992 AcceptedAugust 17, 1992 CosmNzo, J. P., and LBp, R. E., Jn. 1993. Cryoprotectantproduction capacityof the freeze-tolerantwood frog, Rana sylvatica.Can. J. Zool. 7I: 7l-75. Freezingsurvival of the wood frog (Rana sylvatica)is enhancedby the synthesisof the cryoprotectantglucose, via liver glycogenolysis.Because the quantityof glucosemobilized during freezingbears significantly on the limit of freezetolerance, we investigated the relationship between the quantity of liver glycogen and the capacity for cryoprotectant synthesis. We successfullyaugmented natural levels of liver glycogenby injecting cold-conditionedwood frogs with glucose.Groups of 8 frogs having mean liver glycogenconcentrations of 554 + 57 (SE), 940 + 57, and 1264 + 66 pmollg catabolized98.7, 83.4, and 52.8%, respectively,of their glycogenreserves during 24 h of freezingto -2.5'C. Glucoseconcentrations con- comitantly increased,reaching 2l + 3, 102 + 23, and 119 + 14 pmollg, respectively,in the liver, and 15 * 3,42 + 5, and 6l * 5 pcmol/ml-, respectively, in the blood. Becausethe capacity for cryoprotectant synthesisdepends on the amount of liver glycogen,the greatestrisk of freezinginjury likely occursduring spring,when glycogenreserves are minimal. Non- glucoseosmolites were important in the wood frog's cryoprotectantsystem, especially in frogs having low glycogenlevels. Presumably the natural variation in cryoprotectant synthesis capacity among individuals and populations of R. sylvatica chiefly reflectsdifferences in glycogenreserves; however, environmental,physiological, and geneticfactors likely are also involved. CosmNzo, J. P., et LEs, R. E., Jn. -

Oceans of Archaea Abundant Oceanic Crenarchaeota Appear to Derive from Thermophilic Ancestors That Invaded Low-Temperature Marine Environments
Oceans of Archaea Abundant oceanic Crenarchaeota appear to derive from thermophilic ancestors that invaded low-temperature marine environments Edward F. DeLong arth’s microbiota is remarkably per- karyotes), Archaea, and Bacteria. Although al- vasive, thriving at extremely high ternative taxonomic schemes have been recently temperature, low and high pH, high proposed, whole-genome and other analyses E salinity, and low water availability. tend to support Woese’s three-domain concept. One lineage of microbial life in par- Well-known and cultivated archaea generally ticular, the Archaea, is especially adept at ex- fall into several major phenotypic groupings: ploiting environmental extremes. Despite their these include extreme halophiles, methanogens, success in these challenging habitats, the Ar- and extreme thermophiles and thermoacido- chaea may now also be viewed as a philes. Early on, extremely halo- cosmopolitan lot. These microbes philic archaea (haloarchaea) were exist in a wide variety of terres- first noticed as bright-red colonies trial, freshwater, and marine habi- Archaea exist in growing on salted fish or hides. tats, sometimes in very high abun- a wide variety For many years, halophilic isolates dance. The oceanic Marine Group of terrestrial, from salterns, salt deposits, and I Crenarchaeota, for example, ri- freshwater, and landlocked seas provided excellent val total bacterial biomass in wa- marine habitats, model systems for studying adap- ters below 100 m. These wide- tations to high salinity. It was only spread Archaea appear to derive sometimes in much later, however, that it was from thermophilic ancestors that very high realized that these salt-loving invaded diverse low-temperature abundance “bacteria” are actually members environments. -

Sperm Cryopreservation: a Review on Current Molecular Cryobiology and Advanced Approaches
1 RBMO VOLUME 00 ISSUE 0 2018 REVIEW Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches BIOGRAPHY Abdolhossein Shahverdi is Professor of Embryology and scientific director of the Sperm Biology Group at the Royan Institute in Tehran. His main research interests are fertility preservation, reproductive epigenetic and germ cell biology. With over 20 years’ experience in reproductive biology, he has published more than 120 international scientific papers. Maryam Hezavehei1,2, Mohsen Sharafi3,*, Homa Mohseni Kouchesfahani2, Ralf Henkel4, Ashok Agarwal5, Vahid Esmaeili1, Abdolhossein Shahverdi1,* KEY MESSAGE Understanding the new aspects of sperm cryobiology, such as epigenetic and proteomic modulation, as well as novel techniques, is essential for clinical applications and the improvement of ART protocols. Long-term follow-up studies on the resultant offspring obtained from cryopreserved spermatozoa is recommended for future studies. ABSTRACT The cryopreservation of spermatozoa was introduced in the 1960s as a route to fertility preservation. Despite the extensive progress that has been made in this field, the biological and biochemical mechanisms involved in cryopreservation have not been thoroughly elucidated to date. Various factors during the freezing process, including sudden temperature changes, ice formation and osmotic stress, have been proposed as reasons for poor sperm quality post-thaw. Little is known regarding the new aspects of sperm cryobiology, such as epigenetic and proteomic modulation of sperm and trans-generational effects of sperm freezing. This article reviews recent reports on molecular and cellular modifications of spermatozoa during cryopreservation in order to collate the existing understanding in this field. The aim is to discuss current freezing techniques and novel strategies that have been developed for sperm protection against cryo-damage, as well as evaluating the probable effects of sperm freezing on offspring health. -

Effects of Sample Treatments on Genome Recovery Via Single-Cell Genomics
The ISME Journal (2014) 8, 2546–2549 & 2014 International Society for Microbial Ecology All rights reserved 1751-7362/14 www.nature.com/ismej SHORT COMMUNICATION Effects of sample treatments on genome recovery via single-cell genomics Scott Clingenpeel1, Patrick Schwientek1, Philip Hugenholtz2 and Tanja Woyke1 1DOE Joint Genome Institute, Walnut Creek, CA, USA and 2Australian Centre for Ecogenomics, School of Chemistry and Molecular Biosciences, University of Queensland, Brisbane, Queensland, Australia Single-cell genomics is a powerful tool for accessing genetic information from uncultivated microorganisms. Methods of handling samples before single-cell genomic amplification may affect the quality of the genomes obtained. Using three bacterial strains we show that, compared to cryopreservation, lower-quality single-cell genomes are recovered when the sample is preserved in ethanol or if the sample undergoes fluorescence in situ hybridization, while sample preservation in paraformaldehyde renders it completely unsuitable for sequencing. The ISME Journal (2014) 8, 2546–2549; doi:10.1038/ismej.2014.92; published online 13 June 2014 Relatively little is known about the functioning of genomes from single cells. Three methods of sample complex microbial communities, largely due to the preservation were tested: cryopreservation with difficulty in culturing most microbes (Rappe and 20% glycerol as a cryoprotectant, preservation in Giovannoni, 2003). Although metagenomics can 70% ethanol, and preservation in 4% paraformalde- provide information on the genetic capabilities of hyde. Because paraformaldehyde treatment causes the entire community, it is difficult to connect crosslinks between nucleic acids and proteins, we predicted gene functions to specific organisms using tested cells exposed to 4% paraformaldehyde with metagenomics (Morales and Holben, 2011).