Rhythmicity of Coastal Marine Picoeukaryotes, Bacteria and Archaea Despite Irregular Environmental Perturbations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Arctic Picoeukaryote Micromonas Pusilla Benefits
Biogeosciences Discuss., https://doi.org/10.5194/bg-2018-28 Manuscript under review for journal Biogeosciences Discussion started: 5 February 2018 c Author(s) 2018. CC BY 4.0 License. 1 The Arctic picoeukaryote Micromonas pusilla benefits 2 synergistically from warming and ocean acidification 3 4 Clara J. M. Hoppe1,2*, Clara M. Flintrop1,3 and Björn Rost1 5 6 1 Marine Biogeosciences, Alfred Wegener Institute – Helmholtz Centre for Polar and Marine 7 Research, 27570 Bremerhaven, Germany 8 2 Norwegian Polar Institute, 9296 Tromsø, Norway 9 3 MARUM, 28359 Bremen, Germany 10 11 *Correspondence to: Clara J. M. Hoppe ([email protected] 12 13 14 15 Abstract 16 In the Arctic Ocean, climate change effects such as warming and ocean acidification (OA) are 17 manifesting faster than in other regions. Yet, we are lacking a mechanistic understanding of the 18 interactive effects of these drivers on Arctic primary producers. In the current study, one of the 19 most abundant species of the Arctic Ocean, the prasinophyte Micromonas pusilla, was exposed 20 to a range of different pCO2 levels at two temperatures representing realistic scenarios for 21 current and future conditions. We observed that warming and OA synergistically increased 22 growth rates at intermediate to high pCO2 levels. Furthermore, elevated temperatures shifted 23 the pCO2-optimum of biomass production to higher levels. Based on changes in cellular 24 composition and photophysiology, we hypothesise that the observed synergies can be explained 25 by beneficial effects of warming on carbon fixation in combination with facilitated carbon 26 acquisition under OA. Our findings help to understand the higher abundances of picoeukaryotes 27 such as M. -

Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide
antioxidants Review Anoxygenic Photosynthesis in Photolithotrophic Sulfur Bacteria and Their Role in Detoxication of Hydrogen Sulfide Ivan Kushkevych 1,* , Veronika Bosáková 1,2 , Monika Vítˇezová 1 and Simon K.-M. R. Rittmann 3,* 1 Department of Experimental Biology, Faculty of Science, Masaryk University, 62500 Brno, Czech Republic; [email protected] (V.B.); [email protected] (M.V.) 2 Department of Biology, Faculty of Medicine, Masaryk University, 62500 Brno, Czech Republic 3 Archaea Physiology & Biotechnology Group, Department of Functional and Evolutionary Ecology, Universität Wien, 1090 Vienna, Austria * Correspondence: [email protected] (I.K.); [email protected] (S.K.-M.R.R.); Tel.: +420-549-495-315 (I.K.); +431-427-776-513 (S.K.-M.R.R.) Abstract: Hydrogen sulfide is a toxic compound that can affect various groups of water microorgan- isms. Photolithotrophic sulfur bacteria including Chromatiaceae and Chlorobiaceae are able to convert inorganic substrate (hydrogen sulfide and carbon dioxide) into organic matter deriving energy from photosynthesis. This process takes place in the absence of molecular oxygen and is referred to as anoxygenic photosynthesis, in which exogenous electron donors are needed. These donors may be reduced sulfur compounds such as hydrogen sulfide. This paper deals with the description of this metabolic process, representatives of the above-mentioned families, and discusses the possibility using anoxygenic phototrophic microorganisms for the detoxification of toxic hydrogen sulfide. Moreover, their general characteristics, morphology, metabolism, and taxonomy are described as Citation: Kushkevych, I.; Bosáková, well as the conditions for isolation and cultivation of these microorganisms will be presented. V.; Vítˇezová,M.; Rittmann, S.K.-M.R. -

Limits of Life on Earth Some Archaea and Bacteria
Limits of life on Earth Thermophiles Temperatures up to ~55C are common, but T > 55C is Some archaea and bacteria (extremophiles) can live in associated usually with geothermal features (hot springs, environments that we would consider inhospitable to volcanic activity etc) life (heat, cold, acidity, high pressure etc) Thermophiles are organisms that can successfully live Distinguish between growth and survival: many organisms can survive intervals of harsh conditions but could not at high temperatures live permanently in such conditions (e.g. seeds, spores) Best studied extremophiles: may be relevant to the Interest: origin of life. Very hot environments tolerable for life do not seem to exist elsewhere in the Solar System • analogs for extraterrestrial environments • `extreme’ conditions may have been more common on the early Earth - origin of life? • some unusual environments (e.g. subterranean) are very widespread Extraterrestrial Life: Spring 2008 Extraterrestrial Life: Spring 2008 Grand Prismatic Spring, Yellowstone National Park Hydrothermal vents: high pressure in the deep ocean allows liquid water Colors on the edge of the at T >> 100C spring are caused by different colonies of thermophilic Vents emit superheated water (300C or cyanobacteria and algae more) that is rich in minerals Hottest water is lifeless, but `cooler’ ~50 species of such thermophiles - mostly archae with some margins support array of thermophiles: cyanobacteria and anaerobic photosynthetic bacteria oxidize sulphur, manganese, grow on methane + carbon monoxide etc… Sulfolobus: optimum T ~ 80C, minimum 60C, maximum 90C, also prefer a moderately acidic pH. Live by oxidizing sulfur Known examples can grow (i.e. multiply) at temperatures which is abundant near hot springs. -

2018 Strassert JFH, Hehenberger E, Del Campo J, Okamoto N, Kolisko M
2018 Strassert JFH, Hehenberger E, del Campo J, Okamoto N, Kolisko M, Richards TA, Worden AZ, Santoro AE & PJ Keeling. Phylogeny, evidence for a cryptic plastid, and distribution of Chytriodinium parasites (Dinophyceae) infecting copepods. Journal of Eukaryotic Microbiology. https://doi.org/10.1111/jeu.12701 Joo S, Wang MH, Lui G, Lee J, Barnas A, Kim E, Sudek S, Worden AZ & JH Lee. Common ancestry of heterodimerizing TALE homeobox transcription factors across Metazoa and Archaeplastida. BMC Biology. 16:136. doi: 10.1186/s12915-018-0605-5 Bachy C, Charlesworth CJ, Chan AM, Finke JF, Wong C-H, Wei C-L, Sudek S, Coleman ML, Suttle CA & AZ Worden. Transcriptional responses of the marine green alga Micromonas pusilla and an infecting prasinovirus under different phosphate conditions. Environmental Microbiology. Vol 20:2898-2912. Guo J, Wilken S, Jimenez V, Choi CJ, Ansong CK, Dannebaum R, Sudek L, Milner D, Bachy C, Reistetter EN, Elrod VA, Klimov D, Purvine SO, Wei C-L, Kunde-Ramamoorthy G, Richards TA, Goodenough U, Smith RD, Callister SJ & AZ Worden. Specialized proteomic responses and an ancient photoprotection mechanism sustain marine green algal growth during phosphate limitation. Nature Microbiology. Vol 3:781–790. Okamoto N, Gawryluk RMR, del Campo J, Strassert JFH, Lukeš J, Richards TA, Worden AZ, Santoro AE & PJ Keeling. A revised taxonomy of diplonemids Including the Eupelagonemidae n. fam. and a Type Species, Eupelagonema oceanica n. gen. & sp. The Journal of Eukaryotic Microbiology. https://doi.org/10.1111/jeu.12679 Orsi WD, Wilken S, del Campo J, Heger T, James E, Richards TA, Keeling PJ, Worden AZ & AE. -

PROTISTS Shore and the Waves Are Large, Often the Largest of a Storm Event, and with a Long Period
(seas), and these waves can mobilize boulders. During this phase of the storm the rapid changes in current direction caused by these large, short-period waves generate high accelerative forces, and it is these forces that ultimately can move even large boulders. Traditionally, most rocky-intertidal ecological stud- ies have been conducted on rocky platforms where the substrate is composed of stable basement rock. Projec- tiles tend to be uncommon in these types of habitats, and damage from projectiles is usually light. Perhaps for this reason the role of projectiles in intertidal ecology has received little attention. Boulder-fi eld intertidal zones are as common as, if not more common than, rock plat- forms. In boulder fi elds, projectiles are abundant, and the evidence of damage due to projectiles is obvious. Here projectiles may be one of the most important defi ning physical forces in the habitat. SEE ALSO THE FOLLOWING ARTICLES Geology, Coastal / Habitat Alteration / Hydrodynamic Forces / Wave Exposure FURTHER READING Carstens. T. 1968. Wave forces on boundaries and submerged bodies. Sarsia FIGURE 6 The intertidal zone on the north side of Cape Blanco, 34: 37–60. Oregon. The large, smooth boulders are made of serpentine, while Dayton, P. K. 1971. Competition, disturbance, and community organi- the surrounding rock from which the intertidal platform is formed zation: the provision and subsequent utilization of space in a rocky is sandstone. The smooth boulders are from a source outside the intertidal community. Ecological Monographs 45: 137–159. intertidal zone and were carried into the intertidal zone by waves. Levin, S. A., and R. -

Spatial Variability of Picoeukaryotic Communities in the Mariana Trench Hongmei Jing1, Yue Zhang1,2, Yingdong Li3, Wenda Zhu1,2 & Hongbin Liu 3
www.nature.com/scientificreports OPEN Spatial Variability of Picoeukaryotic Communities in the Mariana Trench Hongmei Jing1, Yue Zhang1,2, Yingdong Li3, Wenda Zhu1,2 & Hongbin Liu 3 Picoeukaryotes play prominent roles in the biogeochemical cycles in marine ecosystems. However, their Received: 14 June 2018 molecular diversity studies have been confned in marine surface waters or shallow coastal sediments. Accepted: 5 October 2018 Here, we investigated the diversity and metabolic activity of picoeukaryotic communities at depths Published: xx xx xxxx ranging from the surface to the abyssopelagic zone in the western Pacifc Ocean above the north and south slopes of the Mariana Trench. This was achieved by amplifying and sequencing the V4 region of both 18S ribosomal DNA and cDNA using Illumina HiSeq sequencing. Our study revealed: (1) Four super-groups (i.e., Alveolata, Opisthokonta, Rhizaria and Stramenopiles) dominated the picoeukaryote assemblages through the water column, although they accounted for diferent proportions at DNA and cDNA levels. Our data expand the deep-sea assemblages from current bathypelagic to abyssopelagic zones. (2) Using the cDNA-DNA ratio as a proxy of relative metabolic activity, the highest activity for most subgroups was usually found in the mesopelagic zone; and (3) Population shift along the vertical scale was more prominent than that on the horizontal diferences, which might be explained by the sharp physicochemical gradients along the water depths. Overall, our study provides a better understanding of the diversity and metabolic activity of picoeukaryotes in water columns of the deep ocean in response to varying environmental conditions. Marine picoeukaryotes, (i.e., picoplanktonic eukaryotes of <2 μm in size), are capable of photosynthetic, hetero- trophic and mixotrophic metabolisms1. -

Identification of Associations Between Bacterioplankton and Photosynthetic Picoeukaryotes in Coastal Waters
fmicb-07-00339 March 22, 2016 Time: 11:12 # 1 ORIGINAL RESEARCH published: 22 March 2016 doi: 10.3389/fmicb.2016.00339 Identification of Associations between Bacterioplankton and Photosynthetic Picoeukaryotes in Coastal Waters Hanna M. Farnelid1,2*, Kendra A. Turk-Kubo1 and Jonathan P. Zehr1 1 Ocean Sciences Department, University of California at Santa Cruz, Santa Cruz, CA, USA, 2 Centre for Ecology and Evolution in Microbial Model Systems, Linnaeus University, Kalmar, Sweden Photosynthetic picoeukaryotes are significant contributors to marine primary productivity. Associations between marine bacterioplankton and picoeukaryotes frequently occur and can have large biogeochemical impacts. We used flow cytometry to sort cells from seawater to identify non-eukaryotic phylotypes that are associated with photosynthetic picoeukaryotes. Samples were collected at the Santa Cruz wharf on Monterey Bay, CA, USA during summer and fall, 2014. The phylogeny of associated microbes was assessed through 16S rRNA gene amplicon clone and Illumina MiSeq libraries. The most frequently detected bacterioplankton phyla Edited by: within the photosynthetic picoeukaryote sorts were Proteobacteria (Alphaproteobacteria Xavier Mayali, and Gammaproteobacteria) and Bacteroidetes. Intriguingly, the presence of free-living Lawrence Livermore National Laboratory, USA bacterial genera in the photosynthetic picoeukaryote sorts could suggest that some Reviewed by: of the photosynthetic picoeukaryotes were mixotrophs. However, the occurrence of Cécile Lepère, bacterial sequences, which were not prevalent in the corresponding bulk seawater Blaise Pascal University, France Manuela Hartmann, samples, indicates that there was also a selection for specific OTUs in association with National Oceanography Centre, UK photosynthetic picoeukaryotes suggesting specific functional associations. The results Michael Morando, show that diverse bacterial phylotypes are found in association with photosynthetic University of Southern California, USA picoeukaryotes. -

Algae and Cyanobacteria in Coastal and Estuarine Waters
CHAPTER 7 Algae and cyanobacteria in coastal and estuarine waters n coastal and estuarine waters, algae range from single-celled forms to the seaweeds. ICyanobacteria are organisms with some characteristics of bacteria and some of algae. They are similar in size to the unicellular algae and, unlike other bacteria, contain blue-green or green pigments and are able to perform photosynthesis; thus, they are also termed blue-green algae. Algal blooms in the sea have occurred throughout recorded history but have been increasing during recent decades (Anderson, 1989; Smayda, 1989a; Hallegraeff, 1993). In several areas (e.g., the Baltic and North seas, the Adriatic Sea, Japanese coastal waters and the Gulf of Mexico), algal blooms are a recurring phe- nomenon. The increased frequency of occurrence has accompanied nutrient enrich- ment of coastal waters on a global scale (Smayda, 1989b). Blooms of non-toxic phytoplankton species and mass occurrences of macro-algae can affect the amenity value of recreational waters due to reduced transparency, dis- coloured water and scum formation. Furthermore, bloom degradation can be accom- panied by unpleasant odours, resulting in aesthetic problems (see chapter 9). Several human diseases have been reported to be associated with many toxic species of dinoflagellates, diatoms, nanoflagellates and cyanobacteria that occur in the marine environment (CDC, 1997). The effects of these algae on humans are due to some of their constituents, principally algal toxins. Marine algal toxins become a problem primarily because they may concentrate in shellfish and fish that are subsequently eaten by humans (CDR, 1991; Lehane, 2000), causing syndromes known as para- lytic shellfish poisoning (PSP), diarrhetic shellfish poisoning (DSP), amnesic shellfish poisoning (ASP), neurotoxic shellfish poisoning (NSP) and ciguatera fish poisoning (CFP). -
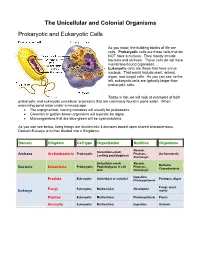
The Unicellular and Colonial Organisms Prokaryotic And
The Unicellular and Colonial Organisms Prokaryotic and Eukaryotic Cells As you know, the building blocks of life are cells. Prokaryotic cells are those cells that do NOT have a nucleus. They mostly include bacteria and archaea. These cells do not have membrane-bound organelles. Eukaryotic cells are those that have a true nucleus. That would include plant, animal, algae, and fungal cells. As you can see, to the left, eukaryotic cells are typically larger than prokaryotic cells. Today in lab, we will look at examples of both prokaryotic and eukaryotic unicellular organisms that are commonly found in pond water. When examining pond water under a microscope… The unpigmented, moving microbes will usually be protozoans. Greenish or golden-brown organisms will typically be algae. Microorganisms that are blue-green will be cyanobacteria. As you can see below, living things are divided into 3 domains based upon shared characteristics. Domain Eukarya is further divided into 4 Kingdoms. Domain Kingdom Cell type Organization Nutrition Organisms Absorb, Unicellular-small; Prokaryotic Photsyn., Archaeacteria Archaea Archaebacteria Lacking peptidoglycan Chemosyn. Unicellular-small; Absorb, Bacteria, Prokaryotic Peptidoglycan in cell Photsyn., Bacteria Eubacteria Cyanobacteria wall Chemosyn. Ingestion, Eukaryotic Unicellular or colonial Protozoa, Algae Protista Photosynthesis Fungi, yeast, Fungi Eukaryotic Multicellular Absorption Eukarya molds Plantae Eukaryotic Multicellular Photosynthesis Plants Animalia Eukaryotic Multicellular Ingestion Animals Prokaryotic Organisms – the archaea, non-photosynthetic bacteria, and cyanobacteria Archaea - Microorganisms that resemble bacteria, but are different from them in certain aspects. Archaea cell walls do not include the macromolecule peptidoglycan, which is always found in the cell walls of bacteria. Archaea usually live in extreme, often very hot or salty environments, such as hot mineral springs or deep-sea hydrothermal vents. -

Cyanobacteria Blooms in the Baltic Sea: a Review of Models and Facts
https://doi.org/10.5194/bg-2020-151 Preprint. Discussion started: 19 May 2020 c Author(s) 2020. CC BY 4.0 License. Cyanobacteria Blooms in the Baltic Sea: A Review of Models and Facts Britta Munkes1, Ulrike Löptien1,2, and Heiner Dietze1,2 1GEOMAR, Helmholtz Centre for Ocean Research Kiel, Düsternbrooker Weg 20, D-24105 Kiel, Germany. 2Institute of Geosciences, Christian-Albrechts-University of Kiel, Ludewig-Meyn-Str. 10, 24 118 Kiel, Germany Correspondence: Britta Munkes ([email protected]) Abstract. The ecosystem of the Baltic Sea is endangered by eutrophication. This has triggered expensive international man- agement efforts. Some of these efforts are impeded by natural processes such as nitrogen-fixing cyanobacteria blooms that add bioavailable nitrogen to the already over-fertilised system and thereby enhance primary production, export of organic matter to depth and associated oxygen consumption. Controls of cyanobacteria blooms are not comprehensively understood and this 5 adds to the uncertainty of model-based projections into the warming future of the Baltic Sea. Here we review our current un- derstanding of cyanobacteria bloom dynamics. We summarise published field studies, laboratory experiments and dissect the basic principles ingrained in state-of-the-art coupled ocean-circulation biogeochemical models. 1 Introduction 10 The Baltic Sea is a shallow, brackish and semi-enclosed sea in central Northern Europe. It’s drainage basin is densely populated by around 84 million people. Their footprint exerts pressure on the ecosystem (Unger et al., 2013; Hannerz and Destouni, 2006). One, particularly severe, problem is eutrophication. Antropogenic nutrients enter the Baltic Sea via rivers and air-sea fluxes (Helcom, 2018, 2014). -

About Cyanobacteria BACKGROUND Cyanobacteria Are Single-Celled Organisms That Live in Fresh, Brackish, and Marine Water
About Cyanobacteria BACKGROUND Cyanobacteria are single-celled organisms that live in fresh, brackish, and marine water. They use sunlight to make their own food. In warm, nutrient-rich environments, microscopic cyanobacteria can grow quickly, creating blooms that spread across the water’s surface and may become visible. Because of the color, texture, and location of these blooms, the common name for cyanobacteria is blue-green algae. However, cyanobacteria are related more closely to bacteria than to algae. Cyanobacteria are found worldwide, from Brazil to China, Australia to the United States. In warmer climates, these organisms can grow year-round. Scientists have called cyanobacteria the origin of plants, and have credited cyanobacteria with providing nitrogen fertilizer for rice and beans. But blooms of cyanobacteria are not always helpful. When these blooms become harmful to the environment, animals, and humans, scientists call them cyanobacterial harmful algal blooms (CyanoHABs). Freshwater CyanoHABs can use up the oxygen and block the sunlight that other organisms need to live. They also can produce powerful toxins that affect the brain and liver of animals and humans. Because of concerns about CyanoHABs, which can grow in drinking water and recreational water, the U.S. Environmental Protection Agency (EPA) has added cyanobacteria to its Drinking Water Contaminant Candidate List. This list identifies organisms and toxins that EPA considers to be priorities for investigation. ASSESSING THE IMPACT ON PUBLIC HEALTH Reports of poisonings associated with CyanoHABs date back to the late 1800s. Anecdotal evidence and data from laboratory animal research suggest that cyanobacterial toxins can cause a range of adverse human health effects, yet few studies have explored the links between CyanoHABs and human health. -

Stimulated Bacterioplankton Growth and Selection for Certain Bacterial Taxa in the Vicinity of the Ctenophore Mnemiopsis Leidyi
ORIGINAL RESEARCH ARTICLE published: 16 August 2012 doi: 10.3389/fmicb.2012.00302 Stimulated bacterioplankton growth and selection for certain bacterial taxa in the vicinity of the ctenophore Mnemiopsis leidyi Julie Dinasquet 1,2, Lena Granhag 3,4*andLasse Riemann 2 1 Department of Natural Sciences, Linnaeus University, Kalmar, Sweden 2 Marine Biological Section, University of Copenhagen, Helsingør, Denmark 3 Department of Marine Ecology-Kristineberg, University of Gothenburg, Gothenburg, Sweden 4 Department of Shipping and Marine Technology, Chalmers University of Technology, Gothenburg, Sweden Edited by: Episodic blooms of voracious gelatinous zooplankton, such as the ctenophore Kam W. Tang, Virginia Institute of Mnemiopsis leidyi, affect pools of inorganic nutrients and dissolved organic carbon Marine Science, USA by intensive grazing activities and mucus release. This will potentially influence Reviewed by: bacterioplankton activity and community composition, at least at local scales; however, Hans-Peter Grossart, IGB-Leibniz-Institute of Freshwater available studies on this are scarce. In the present study we examined effects of M. leidyi Ecology and Inland Fisheries, on bacterioplankton growth and composition in incubation experiments. Moreover, we Germany examined community composition of bacteria associated with the surface and gut Samantha L. Bickel, Virginia Institute of M. leidyi. High release of ammonium and high bacterial growth was observed in of Marine Science, USA the treatments with M. leidyi relative to controls. Deep 454 pyrosequencing of 16 S *Correspondence: Lena Granhag, Department of rRNA genes showed specific bacterial communities in treatments with M. leidyi as Marine Ecology-Kristineberg, well as specific communities associated with M. leidyi tissue and gut. In particular, University of Gothenburg, members of Flavobacteriaceae were associated with M.