Diptera: Culicidae: Aedini) Based on Morphological Data from All Life Stages
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Identification Key for Mosquito Species
‘Reverse’ identification key for mosquito species More and more people are getting involved in the surveillance of invasive mosquito species Species name used Synonyms Common name in the EU/EEA, not just professionals with formal training in entomology. There are many in the key taxonomic keys available for identifying mosquitoes of medical and veterinary importance, but they are almost all designed for professionally trained entomologists. Aedes aegypti Stegomyia aegypti Yellow fever mosquito The current identification key aims to provide non-specialists with a simple mosquito recog- Aedes albopictus Stegomyia albopicta Tiger mosquito nition tool for distinguishing between invasive mosquito species and native ones. On the Hulecoeteomyia japonica Asian bush or rock pool Aedes japonicus japonicus ‘female’ illustration page (p. 4) you can select the species that best resembles the specimen. On japonica mosquito the species-specific pages you will find additional information on those species that can easily be confused with that selected, so you can check these additional pages as well. Aedes koreicus Hulecoeteomyia koreica American Eastern tree hole Aedes triseriatus Ochlerotatus triseriatus This key provides the non-specialist with reference material to help recognise an invasive mosquito mosquito species and gives details on the morphology (in the species-specific pages) to help with verification and the compiling of a final list of candidates. The key displays six invasive Aedes atropalpus Georgecraigius atropalpus American rock pool mosquito mosquito species that are present in the EU/EEA or have been intercepted in the past. It also contains nine native species. The native species have been selected based on their morpho- Aedes cretinus Stegomyia cretina logical similarity with the invasive species, the likelihood of encountering them, whether they Aedes geniculatus Dahliana geniculata bite humans and how common they are. -

Zootaxa, New Records of Haemagogus
Zootaxa 1779: 65–68 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Correspondence ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) New records of Haemagogus (Haemagogus) from Northern and Northeastern Brazil (Diptera: Culicidae, Aedini) JERÔNIMO ALENCAR1, FRANCISCO C. CASTRO2, HAMILTON A. O. MONTEIRO2, ORLANDO V. SILVA 2, NICOLAS DÉGALLIER3, CARLOS BRISOLA MARCONDES4*, ANTHONY E. GUIMARÃES1 1Laboratório de Diptera, Departamento de Entomologia, Instituto Oswaldo Cruz, Av. Brasil 4365, CEP: 21045-900 Manguinhos, Rio de Janeiro RJ, Brazil. 2Laboratório de Arbovírus, Instituto Evandro Chagas, Av. Almirante Barroso 492, CEP: 66090-000, Belém, PA, Brazil. 3Institut de Recherche pour le Développement (IRD-UMR182), LOCEAN-IPSL, case 100, 4 Place Jussieu, 75252 Paris Cedex 05, France 4 Departamento de Microbiologia e Parasitologia, Centro de Ciências Biológicas, Universidade Federal de Santa Catarina, 88040- 900 Florianópolis, Santa Catarina, Brazil Haemagogus (Haemagogus) is restricted mostly to the Neotropical Region, including Central America, South America and islands (Arnell, 1973). Of the 24 recognized species of this subgenus, 15 occur in South America, including the Anti- lles. However, the centre of distribution of the genus Haemagogus is Central America, where 19 of the 28 species (including four species of the subgenus Conopostegus Zavortink [1972]) occur (Arnell, 1973). Haemagogus (Hag.) includes species with great significance as vectors of Yellow Fever (YF) virus and other arbovi- rus, both experimentally (Waddell, 1949) and in the field (Vasconcelos, 2003). During entomological surveys from 1982 to 2004, the Arbovirus Laboratory of Evandro Chagas Institute obtained specimens of Haemagogus from several localities not reported in the literature. New records are listed in Table 1 and study localities shown on Figure 1. -

Diptera: Culicidae: Aedini) Into New Geographic Areas
European Mosquito Bulletin, 27 (2009), 10-17. Journal of the European Mosquito Control Association ISSN 1460-6127; w.w.w.e-m-b.org First published online 1 October 2009 Recent introductions of aedine species (Diptera: Culicidae: Aedini) into new geographic areas John F. Reinert Center for Medical, Agricultural and Veterinary Entomology (CMAVE), United States Department of Agriculture, Agricultural Research Service, 1600/1700 S.W. 23rd Drive, Gainesville, FL 32608-1067, USA, Email: [email protected]. Abstract Information on introductions to new geographic areas of species in the aedine generic-level taxa Aedimorphus, Finlaya, Georgecraigius, Halaedes, Howardina, Hulecoeteomyia, Rampamyia, Stegomyia, Tanakaius and Verrallina is provided. Key words: Aedimorphus, Finlaya, Georgecraigius atropalpus, Halaedes australis, Howardina bahamensis, Hulecoeteomyia japonica japonica, Rampamyia notoscripta, Stegomyia aegypti, Stegomyia albopicta, Tanakaius togoi, Verrallina Introduction World. Dyar (1928), however, notes that there are no nearly related species in As indicated in the series of papers on the American continent, but many such the phylogeny and classification of in the Old World, especially in Africa, mosquitoes in tribe Aedini (Reinert et and he considered that it was probably al., 2004, 2006, 2008), some aedine the African continent from which the species have been introduced into new species originated”. Christophers also geographical areas in recent times. noted that “The species is almost the Species of Aedini found outside of their only, if not the only, mosquito that, with natural ranges are listed below with their human agency, is spread around the literature citations. whole globe. But in spite of this wide zonal diffusion its distribution is very Introductions of Aedine Species to strictly limited by latitude and as far as New Areas present records go it very rarely occurs beyond latitudes of 45o N. -

Diptera: Culicidae) and Their Received: 23-09-2014
Journal of Entomology and Zoology Studies 2014; 2 (5): 134-137 ISSN 2320-7078 New record of Aedes vittatus and Culiseta JEZS 2014; 2 (5): 134-137 © 2014 JEZS subochrea (Diptera: Culicidae) and their Received: 23-09-2014 Accepted: 06-10-2014 distribution from Shadegan Wetland, South Hassan Nasirian Western Iran Department of Medical Entomology and Vector Control, School of Public Health, Tehran Hassan Nasirian, Sayyed Mohammad Taghi Sadeghi, Babak University of Medical Sciences, Tehran, Iran Vazirianzadeh, Sayyed Hassan Moosa-Kazemi Sayyed Mohammad Taghi Abstract Sadeghi This study was conducted in Shadegan International wetland area, south western Iran. Mosquito larvae Department of Medical were collected from an urban site in the east of the wetland, using dipping method in December 2011. Entomology and Vector Control, Mosquito larvae were identified using the morphology-based keys. The water quality parameters were School of Public Health, Tehran measured during mosquito larvae collection using HQ40d Portable Multi-Parameter Meter, titration University of Medical Sciences, method and turbidity meter from the late October to late December 2011. A total of 1071 larvae were Tehran, Iran collected. Four species belonging to four genera including Aedes vittatus (Bigot), Culex pipiens Linnaeus, Culiseta subochrea (Edwards) and Ochlerotatus (=Aedes) caspius (Pallas) sensu lato were identified and Babak Vazirianzadeh their distribution and some other aspects were reviewed and discussed. Recorded parameters in the present Health Research Institute, study indicated that the water quality of the studied area was in a poor condition according to Infectious and Tropical Diseases Environmental Protection Agency and World Health Organization water quality standards (p < 0.05). -

Classification of Mosquitoes in Tribe Aedini 925
FORUM Classification of Mosquitoes in Tribe Aedini (Diptera: Culicidae): Paraphylyphobia, and Classification Versus Cladistic Analysis HARRY M. SAVAGE Centers for Disease Control and Prevention, P.O. Box 2087, Fort Collins, CO 80522 J. Med. Entomol. 42(6): 923Ð927 (2005) Downloaded from https://academic.oup.com/jme/article/42/6/923/886357 by guest on 29 September 2021 ABSTRACT Many mosquito species are important vectors of human and animal diseases, and others are important nuisance species. To facilitate communication and information exchange among pro- fessional groups interested in vector-borne diseases, it is essential that a stable nomenclature be maintained. For the Culicidae, easily identiÞable genera based on morphology are an asset. Major changes in generic concept, the elevation of 32 subgenera within Aedes to generic status, and changes in hundreds of species names proposed in a recent article demand consideration by all parties interested in mosquito-borne diseases. The entire approach to Aedini systematics of these authors was ßawed by an inordinate fear of paraphyletic taxa or Paraphylyphobia, and their inability to distinguish between classiÞcation and cladistic analysis. Taxonomists should refrain from making taxonomic changes based on preliminary data, and they should be very selective in assigning generic names to only the most important and well-deÞned groups of species. KEY WORDS Aedini classiÞcation, Aedes, Ochlerotatus, paraphylyphobia, mosquito classiÞcation MANY MOSQUITO SPECIES IN the tribe Aedini, a cosmo- In this communication, I brießy review taxonomic politan group represented by 11 genera and Ϸ1,239 categories in a zoological classiÞcation, the Interna- species, are important vectors of human and animal tional Code of Zoological Nomenclature (the Code), diseases, and many others are of considerable eco- the development of generic concept within the Cu- nomic importance as nuisance or pest species. -

A Multiplex PCR Assay for Six Aedini Species, Including Aedes Albopictus
A Multiplex PCR Assay for Six Aedini Species, Including Aedes albopictus Woo Jun Bang Kyungpook National University College of Natural Sciences Min Hyeok Won Kyungpook National University College of Natural Sciences Seong Tae Cho Kyungpook National University College of Natural Sciences Jihun Ryu Kyungpook National University College of Natural Sciences Kwang Shik Choi ( [email protected] ) Kyungpook National University, Daehak-ro 80, Daegu Methodology Keywords: Aedini species, Aedes albopictus, Internal transcribed spacer 2(ITS2), multiplex PCR assay Posted Date: December 15th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-127286/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Version of Record: A version of this preprint was published at Parasites & Vectors on July 28th, 2021. See the published version at https://doi.org/10.1186/s13071-021-04871-7. Page 1/8 Abstract Background Mosquitoes, as vectors of various human pathogens, are signicant drivers of serious human illness. In particular, those species in the Aedini tribe, which typically transmit dengue virus, Chikungunya fever virus, and Zika virus, are increasing their range because of climate change and international commerce. In order to prevent mosquito-borne disease, accurate mosquito species identication and monitoring are needed. The goal of this work was to develop a rapid and simple molecular diagnostic method for six morphologically similar Aedini species (Aedes avopictus, Aedes albopictus, Ochlerotatus koreicus, Ochlerotatus japonicus, Ochlerotatus togoi, and Ochlerotatus hatorii) in Korea. Methods In total, 109 samples were used in this study. The internal transcribed spacer 2 (ITS2) regions from all six species were amplied, sequenced and analyzed using Mega 6. -

The Taxonomic History of Ochlerotatus Lynch Arribálzaga, 1891 (Diptera: Culicidae)
insects Article The Taxonomic History of Ochlerotatus Lynch Arribálzaga, 1891 (Diptera: Culicidae) Lílian Ferreira de Freitas * and Lyric C. Bartholomay Department of Pathobiological Sciences, School of Veterinary Medicine, University of Wisconsin–Madison, Madison, WI 53706, USA; [email protected] * Correspondence: [email protected] Simple Summary: Mosquitoes are an extremely diverse group of aquatic insects, distributed over all continents except Antarctica. The mosquitoes belonging to the subgenus called Ochlerotatus, as understood by Reinert et al., comprise a group of several species which are endemic to the Americas, some of which are important vectors of human and animal pathogens. However, this group is characteristically undefined, i.e., no sets of characters define the group, and this presents a major challenge for the understanding of the evolutionary tree of the mosquitoes. This work underscores and contextualizes the complex taxonomic history of the group, reveals the major challenges we face in order to resolve the definition of this group, and presents a path forward for its successful revision. Abstract: A review of all taxonomic actions within the subgenus Ochlerotatus Lynch Arribálzaga, 1891 (Diptera: Culicidae) sensu Reinert et al. (2008) is provided. In particular, the complex historical taxo- nomic treatment of the type species of this group is dissected and explained in detail. Additionally, current challenges with the definition of the subgenus and its constituents are discussed, as are the requisite steps for a successful revision of the taxon. Going forward, we conclude that a taxonomic Citation: Ferreira de Freitas, L.; revision of the species should include a neotype designation for Ochlerotatus scapularis (Rondani, Bartholomay, L.C. -
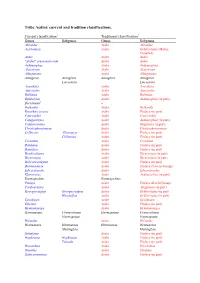
Tribe Aedini: Current and Tradition Classifications
Tribe Aedini: current and tradition classifications. Current classification1 Traditional classification2 Genus Subgenus Genus Subgenus Abraedes Aedes Abraedes Acartomyia Aedes Ochlerotatus (Mariae Complex) Aedes Aedes Aedes “Aedes” sensu auctorum Aedes Aedes Aedimorphus Aedes Aedimorphus Alanstonea Aedes Alanstonea Albuginosus Aedes Albuginosus Armigeres Armigeres Armigeres Armigeres Leicesteria Leicesteria Ayurakitia Aedes Ayurakitia Aztecaedes Aedes Aztecaedes Belkinius Aedes Belkinius Bifidistylus Aedes Aedimorphus (in part) Borichinda3 – Bothaella Aedes Bothaella Bruceharrisonius Aedes Finlaya (in part) Cancraedes Aedes Cancraedes Catageiomyia Aedes Aedimorphus (in part) Catatassomyia Aedes Stegomyia (in part) Christophersiomyia Aedes Christophersiomyia Collessius Alloeomyia Aedes Finlaya (in part) Collessius Aedes Finlaya (in part) Cornetius Aedes Cornetius Dahliana Aedes Finlaya (in part) Danielsia Aedes Finlaya (in part) Dendroskusea Aedes Diceromyia (in part) Diceromyia Aedes Diceromyia (in part) Dobrotworskyius Aedes Finlaya (in part) Downsiomyia Aedes Finlaya (Niveus Group) Edwardsaedes Aedes Edwardsaedes Elpeytonius Aedes Aedimorphus (in part) Eretmapodites Eretmapodites Finlaya Aedes Finlaya (Kochi Group) Fredwardsius Aedes Stegomyia (in part) Georgecraigius Georgecraigius Aedes Ochlerotatus (in part) Horsfallius Aedes Ochlerotatus (in part) Geoskusea Aedes Geoskusea Gilesius Aedes Finlaya (in part) Gymnometopa Aedes Gymnometopa Haemagogus Conopostegus Haemagogus Conopostegus Haemagogus Haemagogus Halaedes Aedes Halaedes Heizmannia -

Fly Times Issue 41, October 2008
FLY TIMES ISSUE 41, October, 2008 Stephen D. Gaimari, editor Plant Pest Diagnostics Branch California Department of Food & Agriculture 3294 Meadowview Road Sacramento, California 95832, USA Tel: (916) 262-1131 FAX: (916) 262-1190 Email: [email protected] Welcome to the latest issue of Fly Times! Let me first thank everyone for sending in such interesting articles – I hope you all enjoy reading it as much as I enjoyed putting it together! With that, please let me encourage all of you to consider contributing articles that may be of interest to the Diptera community. Fly Times offers a great forum to report on your research activities and to make requests for taxa being studied, as well as to report interesting observations about flies, to discuss new and improved methods, to advertise opportunities for dipterists, and to report on or announce meetings relevant to the community. This is also a great place to report on your interesting (and hopefully fruitful) collecting activities! The electronic version of the Fly Times continues to be hosted on the North American Dipterists Society website at http://www.nadsdiptera.org/News/FlyTimes/Flyhome.htm. The Diptera community would greatly appreciate your independent contributions to this newsletter. For this issue, I want to again thank all the contributors for sending me so many great articles! That said, we need even more reports on trips, collections, methods, updates, and anything else you can think of about flies, with all the associated digital images you wish to provide. Feel free to share your opinions or provide ideas on how to improve the newsletter (I am very happy to hear ways that I can enhance it!). -

Arthropod-Borne Viruses Arthropod-Borne • Rebekah C
Arthropod-Borne Viruses • Rebekah C. Kading, Aaron C Brault Beckham David and J. Arthropod-Borne Viruses The Outbreak Edition Edited by Rebekah C. Kading, Aaron C Brault and J. David Beckham Printed Edition of the Special Issue Published in Tropical Medicine and Infectious Disease www.mdpi.com/journal/tropicalmed Arthropod-Borne Viruses Arthropod-Borne Viruses: The Outbreak Edition Editors Rebekah C. Kading Aaron C Brault J. David Beckham MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade • Manchester • Tokyo • Cluj • Tianjin Editors Rebekah C. Kading Aaron C Brault Colorado State University Centers for Disease Control and Prevention USA USA J. David Beckham University of Colorado School of Medicine USA Editorial Office MDPI St. Alban-Anlage 66 4052 Basel, Switzerland This is a reprint of articles from the Special Issue published online in the open access journal Tropical Medicine and Infectious Disease (ISSN 2414-6366) (available at: https://www.mdpi.com/ journal/tropicalmed/special issues/Arthropod Borne Viruses). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: LastName, A.A.; LastName, B.B.; LastName, C.C. Article Title. Journal Name Year, Article Number, Page Range. ISBN 978-3-03943-348-3 (Hbk) ISBN 978-3-03943-349-0 (PDF) c 2020 by the authors. Articles in this book are Open Access and distributed under the Creative Commons Attribution (CC BY) license, which allows users to download, copy and build upon published articles, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book as a whole is distributed by MDPI under the terms and conditions of the Creative Commons license CC BY-NC-ND. -

Primera Cita De Aedes (Fredwardsius) Vittatus (Bigot, 1861) (Diptera: Culicidae) En Galicia, Mediante El Proyecto Mosquito Alert
Anales de Biología 40: 41-45, 2018 ARTÍCULO DOI: http://dx.doi.org/10.6018/analesbio.40.05 Ciencia ciudadana y biodiversidad: primera cita de Aedes (Fredwardsius) vittatus (Bigot, 1861) (Diptera: Culicidae) en Galicia, mediante el proyecto Mosquito Alert Roger Eritja1, Marga Rubido-Bará2, Sarah Delacour-Estrella3, Mikel Bengoa4, Ignacio Ruiz-Arrondo5 & Comunidad Mosquito Alert6 1 CREAF, Cerdanyola del Vallès, 08193 Barcelona, España. 2 Departamento de Biología Vegetal y Ciencia del Suelo, Universidad de Vigo, 36310 Vigo, España. 3 Departamento de Patología Animal, Facultad de Veterinaria, Universidad de Zaragoza, 50013 Zaragoza, España. 4 Consultoria Moscard Tigre, Palma de Mallorca, 07013 Illes Balears, España. 5 Centro de Rickettsiosis y Enfermedades Transmitidas por Artrópodos Vectores, CIBIR, 26006 Logroño, España. 6 Equipo y ciudadanos que participan activamente en la plataforma Mosquito Alert (www.mosquitoalert.com) Resumen Correspondencia Se expone la primera cita de Aedes (Fredwardsius) vittatus (Bigot, R. Eritja 1861) en la comunidad autónoma de Galicia, conseguida gracias a E-mail: [email protected] la plataforma de ciencia ciudadana Mosquito Alert. Aun cuando Recibido: 8 diciembre 2017 este proyecto está enfocado hacia la detección y seguimiento de Aceptado: 19 febrero 2018 dos especies de culícidos exóticos invasores: Aedes (Stegomyia) Publicado on-line: 8 marzo 2018 albopictus (Skuse 1894) y Aedes (Stegomyia) aegypti (L.), se evi- dencia la gran capacidad existente para el estudio de la biodiversi- dad así como la -

Mating Systems of Blood-Feeding Flies
27 Oct 2005 13:30 AR ANRV263-EN51-18.tex XMLPublishSM(2004/02/24) P1: OKZ 10.1146/annurev.ento.51.110104.151058 Annu. Rev. Entomol. 2006. 51:413–40 doi: 10.1146/annurev.ento.51.110104.151058 Copyright c 2006 by Annual Reviews. All rights reserved First published online as a Review in Advance on September 21, 2005 MATING SYSTEMS OF BLOOD-FEEDING FLIES Boaz Yuval Department of Entomology, Hebrew University of Jerusalem, Rehovot 76100, Israel; email: [email protected] KeyWords Diptera, sexual behavior, swarm, female choice ■ Abstract The mating system of each species is a unique, dynamic suite of inter- actions between the sexes. In this review I describe these interactions in the families of flies that contain blood-feeding species. A transition from the aerial swarm, with rapid copulae and no direct female choice, to substrate-based systems with lengthy copulae and opportunities for female choice is evident at both a phylogenetic scale and within nematoceran families under specific ecological conditions. Female monogamy is associated with the former, polyandry with the latter. I suggest that the intensity of sexual selection operating on males in systems where the probability of mating is low has favored male ability to control female receptivity. Reproductive success of males is universally correlated to successful foraging for sugar or blood and (in some species and ecological conditions) to body size. Understanding the ecological basis of the mating systems of these flies will help formulate integrative, sustainable, and biologically lucid approaches for their control. INTRODUCTION Every species of sexually reproducing organism manifests a unique mating sys- tem.