GABA Receptors and the Immune System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

Inhibitory Role for GABA in Autoimmune Inflammation
Inhibitory role for GABA in autoimmune inflammation Roopa Bhata,1, Robert Axtella, Ananya Mitrab, Melissa Mirandaa, Christopher Locka, Richard W. Tsienb, and Lawrence Steinmana aDepartment of Neurology and Neurological Sciences and bDepartment of Molecular and Cellular Physiology, Beckman Center for Molecular Medicine, Stanford University, Stanford, CA 94305 Contributed by Richard W. Tsien, December 31, 2009 (sent for review November 30, 2009) GABA, the principal inhibitory neurotransmitter in the adult brain, serum (13). Because actions of exogenous GABA on inflammation has a parallel inhibitory role in the immune system. We demon- and of endogenous GABA on phasic synaptic inhibition both strate that immune cells synthesize GABA and have the machinery occur at millimolar concentrations (5, 8, 9), we hypothesized that for GABA catabolism. Antigen-presenting cells (APCs) express local mechanisms may also operate in the peripheral immune functional GABA receptors and respond electrophysiologically to system to enhance GABA levels near the inflammatory focus. We GABA. Thus, the immune system harbors all of the necessary first asked whether immune cells have synthetic machinery to constituents for GABA signaling, and GABA itself may function as produce GABA by Western blotting for GAD, the principal syn- a paracrine or autocrine factor. These observations led us to ask thetic enzyme. We found significant amounts of a 65-kDa subtype fl further whether manipulation of the GABA pathway in uences an of GAD (GAD-65) in dendritic cells (DCs) and lower levels in animal model of multiple sclerosis, experimental autoimmune macrophages (Fig. 1A). GAD-65 increased when these cells were encephalomyelitis (EAE). Increasing GABAergic activity amelio- stimulated (Fig. -

Midbrain Benzodiazepine-GABA-Serotonin Interactions: Effects on Locomotor Activity in the Rat
Loyola University Chicago Loyola eCommons Dissertations Theses and Dissertations 1982 Midbrain Benzodiazepine-GABA-Serotonin Interactions: Effects on Locomotor Activity in the Rat Stephen Mitchell Sainati Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_diss Part of the Medicine and Health Sciences Commons Recommended Citation Sainati, Stephen Mitchell, "Midbrain Benzodiazepine-GABA-Serotonin Interactions: Effects on Locomotor Activity in the Rat" (1982). Dissertations. 2228. https://ecommons.luc.edu/luc_diss/2228 This Dissertation is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Dissertations by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1982 Stephen Mitchell Sainati MIDBRAIN BE~ZODIAZEPINE-GABA-SEROTONIN INTERACTIONS: EFFECTS ON LOCm10TOR ACTIVITY IN THE RAT by STEPHEN HITCHELL SAINATI A Dissertation Submitted to the Faculty of the Graduate School of Loyola University of Chicago in Partial Fulfillment of the Requirements for the Degree of DOCTOR OF PHILOSOPHY SEPTEMBER 1982 UBFU.. '"\! LOYOLA UN!VERSHY MED·~i.....'. ~'-· CSN'TER ACKNOWLEDGENENTS The author wishes to thank Drs. Anthony J. Castro, Sebastian P. Grossman, Alexander G. Karczmar, and Louis D. van de Kar for their gui dance and assistance in the design and analysis of this dissertation. An especial note of gratitude goes to Dr. Stanley A. Lorens, Director of this dissertation, without whose kind tutelage, counsel and support, this work would not have been possible. Hr. John W. Corliss, Department of Academic Computing Services, must be acknowledged for his assistance in the word processing and printing bf the intermediate and final drafts of this discourse. -

Type of the Paper (Article
Supplementary Material A Proteomics Study on the Mechanism of Nutmeg-induced Hepatotoxicity Wei Xia 1, †, Zhipeng Cao 1, †, Xiaoyu Zhang 1 and Lina Gao 1,* 1 School of Forensic Medicine, China Medical University, Shenyang 110122, P. R. China; lessen- [email protected] (W.X.); [email protected] (Z.C.); [email protected] (X.Z.) † The authors contributed equally to this work. * Correspondence: [email protected] Figure S1. Table S1. Peptide fraction separation liquid chromatography elution gradient table. Time (min) Flow rate (mL/min) Mobile phase A (%) Mobile phase B (%) 0 1 97 3 10 1 95 5 30 1 80 20 48 1 60 40 50 1 50 50 53 1 30 70 54 1 0 100 1 Table 2. Liquid chromatography elution gradient table. Time (min) Flow rate (nL/min) Mobile phase A (%) Mobile phase B (%) 0 600 94 6 2 600 83 17 82 600 60 40 84 600 50 50 85 600 45 55 90 600 0 100 Table S3. The analysis parameter of Proteome Discoverer 2.2. Item Value Type of Quantification Reporter Quantification (TMT) Enzyme Trypsin Max.Missed Cleavage Sites 2 Precursor Mass Tolerance 10 ppm Fragment Mass Tolerance 0.02 Da Dynamic Modification Oxidation/+15.995 Da (M) and TMT /+229.163 Da (K,Y) N-Terminal Modification Acetyl/+42.011 Da (N-Terminal) and TMT /+229.163 Da (N-Terminal) Static Modification Carbamidomethyl/+57.021 Da (C) 2 Table S4. The DEPs between the low-dose group and the control group. Protein Gene Fold Change P value Trend mRNA H2-K1 0.380 0.010 down Glutamine synthetase 0.426 0.022 down Annexin Anxa6 0.447 0.032 down mRNA H2-D1 0.467 0.002 down Ribokinase Rbks 0.487 0.000 -

Compositions and Methods for Selective Delivery of Oligonucleotide Molecules to Specific Neuron Types
(19) TZZ ¥Z_T (11) EP 2 380 595 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 26.10.2011 Bulletin 2011/43 A61K 47/48 (2006.01) C12N 15/11 (2006.01) A61P 25/00 (2006.01) A61K 49/00 (2006.01) (2006.01) (21) Application number: 10382087.4 A61K 51/00 (22) Date of filing: 19.04.2010 (84) Designated Contracting States: • Alvarado Urbina, Gabriel AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Nepean Ontario K2G 4Z1 (CA) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • Bortolozzi Biassoni, Analia Alejandra PT RO SE SI SK SM TR E-08036, Barcelona (ES) Designated Extension States: • Artigas Perez, Francesc AL BA ME RS E-08036, Barcelona (ES) • Vila Bover, Miquel (71) Applicant: Nlife Therapeutics S.L. 15006 La Coruna (ES) E-08035, Barcelona (ES) (72) Inventors: (74) Representative: ABG Patentes, S.L. • Montefeltro, Andrés Pablo Avenida de Burgos 16D E-08014, Barcelon (ES) Edificio Euromor 28036 Madrid (ES) (54) Compositions and methods for selective delivery of oligonucleotide molecules to specific neuron types (57) The invention provides a conjugate comprising nucleuc acid toi cell of interests and thus, for the treat- (i) a nucleic acid which is complementary to a target nu- ment of diseases which require a down-regulation of the cleic acid sequence and which expression prevents or protein encoded by the target nucleic acid as well as for reduces expression of the target nucleic acid and (ii) a the delivery of contrast agents to the cells for diagnostic selectivity agent which is capable of binding with high purposes. -

Transport of Pregabalin Via L-Type Amino Acid Transporter 1 (SLC7A5) in Human Brain Capillary Endothelial Cell Line
Pharm Res (2018) 35: 246 https://doi.org/10.1007/s11095-018-2532-0 RESEARCH PAPER Transport of Pregabalin Via L-Type Amino Acid Transporter 1 (SLC7A5) in Human Brain Capillary Endothelial Cell Line Yu Takahashi1 & Tomohiro Nishimura 1 & Kei Higuchi 2 & Saki Noguchi 1 & Yuma Tega 2 & Toshiki Kurosawa2 & Yo s h i h a r u D e g u c h i 2 & Masatoshi Tomi1 Received: 14 July 2018 /Accepted: 21 October 2018 /Published online: 29 October 2018 # The Author(s) 2018 ABSTRACT Conclusions Our results indicate that LAT1, but not LAT2, Purpose The anti-epileptic drug pregabalin crosses the recognizes pregabalin as a substrate. It is suggested that LAT1 blood-brain barrier (BBB) in spite of its low lipophilicity. mediates pregabalin transport at the BBB. This study was performed to determine whether L-type amino acid transporters (LAT1/SLC7A5 and LAT2/SLC7A8) con- KEYWORDS anti-epileptic drug . blood-brain barrier . tribute to the uptake of pregabalin. L-type amino acid transporter . pregabalin Methods Pregabalin uptake by LATs-transfected HEK293 cells or hCMEC/D3 cells, an in vitro human BBB model, was measured by LC-MS/MS analysis. Expression of LAT1 mRNA in hCMEC/D3 cells was determined by quantitative ABBREVIATIONS RT-PCR analysis. BBB Blood-brain barrier Results Overexpression of LAT1, but not LAT2, in HEK293 BCH 2-Aminobicyclo-(2,2,1)-heptane-2-car- cells significantly increased the cellular uptake of pregabalin, boxylic acid GABA γ and the LAT1-mediated uptake was saturable with a Km of -Aminobutyric acid 0.288 mM. LAT1-mediated amino acid uptake was inhibited hCMEC/D3 cells Human immortalized brain endothelial specifically and almost completely in the presence of 1 mM cell line pregabalin. -

Human Induced Pluripotent Stem Cell–Derived Podocytes Mature Into Vascularized Glomeruli Upon Experimental Transplantation
BASIC RESEARCH www.jasn.org Human Induced Pluripotent Stem Cell–Derived Podocytes Mature into Vascularized Glomeruli upon Experimental Transplantation † Sazia Sharmin,* Atsuhiro Taguchi,* Yusuke Kaku,* Yasuhiro Yoshimura,* Tomoko Ohmori,* ‡ † ‡ Tetsushi Sakuma, Masashi Mukoyama, Takashi Yamamoto, Hidetake Kurihara,§ and | Ryuichi Nishinakamura* *Department of Kidney Development, Institute of Molecular Embryology and Genetics, and †Department of Nephrology, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan; ‡Department of Mathematical and Life Sciences, Graduate School of Science, Hiroshima University, Hiroshima, Japan; §Division of Anatomy, Juntendo University School of Medicine, Tokyo, Japan; and |Japan Science and Technology Agency, CREST, Kumamoto, Japan ABSTRACT Glomerular podocytes express proteins, such as nephrin, that constitute the slit diaphragm, thereby contributing to the filtration process in the kidney. Glomerular development has been analyzed mainly in mice, whereas analysis of human kidney development has been minimal because of limited access to embryonic kidneys. We previously reported the induction of three-dimensional primordial glomeruli from human induced pluripotent stem (iPS) cells. Here, using transcription activator–like effector nuclease-mediated homologous recombination, we generated human iPS cell lines that express green fluorescent protein (GFP) in the NPHS1 locus, which encodes nephrin, and we show that GFP expression facilitated accurate visualization of nephrin-positive podocyte formation in -

Copyrighted Material
Subject index Note: page numbers in italics refer to fi gures, those in bold refer to tables Abbreviations used in subentries abdominal mass patterns 781–782 GERD – gastroesophageal refl ux disease choledochal cysts 1850 perception 781 IBD – infl ammatory bowel disease mesenteric panniculitis 2208 periodicity 708–709 mesenteric tumors 2210 peripheral neurogenic 2429 A omental tumors 2210 pharmacological management 714–717 ABCB1/MDR1 484 , 633 , 634–636 abdominal migrane (AM) 712 , 2428–2429 physical examination 709–710 , 710 ABCB4 abdominal obesity 2230 postprandial 2498–2499 c h o l e s t e r o l g a l l s t o n e s 1817–1818 , abdominal pain 695–722 in pregnancy 842 1819–1820 a c u t e see acute abdominal pain prevalence 695 functions 483–484 , 1813 acute cholecystitis 784 rare/obscure causes 712–713 , 712 intrahepatic cholestasis of pregnancy 848 acute diverticulitis 794–796 , 795 , 1523–1524 , red fl ags 713 low phospholipid-associated 1527 relieving/aggravating factors 709 cholelithiasis 1810 , 1819–1820 , 2393 acute mesenteric ischemia 2492 right lower quadrant 794 m u t a t i o n p h e n o t y p e s 2392 , 2393 a c u t e p a n c r e a t i t i s 795 , 1653 , 1667 right upper quadrant 794 progressive familial intrahepatic cholestasis-3 acute suppurative peritonitis 2196 sickle cell crisis 2419 494 adhesions 713 s i t e 703 , 708 ABCB11 see bile salt export pump (BSEP) AIDS 2200 special pain syndromes 718–720 ABCC2/MRP2 485 , 494 , 870 , 1813 , 2394 , 2395 anxiety 706–707 sphincter of Oddi dysfunction 1877 ABCG2/BCRP 484–485 , 633 -

O'neill2020 Redacted.Pdf (7.617Mb)
This thesis has been submitted in fulfilment of the requirements for a postgraduate degree (e.g. PhD, MPhil, DClinPsychol) at the University of Edinburgh. Please note the following terms and conditions of use: This work is protected by copyright and other intellectual property rights, which are retained by the thesis author, unless otherwise stated. A copy can be downloaded for personal non-commercial research or study, without prior permission or charge. This thesis cannot be reproduced or quoted extensively from without first obtaining permission in writing from the author. The content must not be changed in any way or sold commercially in any format or medium without the formal permission of the author. When referring to this work, full bibliographic details including the author, title, awarding institution and date of the thesis must be given. Functional Characterisation of Spontaneously Active GABAA Receptors in Rat Dentate Gyrus Granule Cells Nathanael O’Neill B.Medsc. (Hons) Doctor of Philosophy The University of Edinburgh 2020 ii Abstract GABAA receptors (GABAARs) are the principal inhibitory neurotransmitter receptors in the adult mammalian central nervous system. GABAARs mediate two forms of inhibition: fast, phasic conductance; and slow, tonic conductance. Tonic conductance arises due to the persistent activation of GABAARs. This persistent activation can occur by GABA-dependent or GABA- independent mechanisms. Low concentrations of ambient GABA activate high affinity GABAARs located outside the synapse – at peri-/extra-synaptic sites – to generate GABA-dependent tonic conductance. In contrast, GABA-independent tonic conductance is generated by GABAARs that activate spontaneously, in the absence of GABA, due to constitutive receptor gating. -
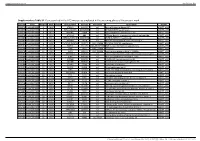
Supplementary Table S1. Genes Printed in the HC5 Microarray Employed in the Screening Phase of the Present Work
Supplementary material Ann Rheum Dis Supplementary Table S1. Genes printed in the HC5 microarray employed in the screening phase of the present work. CloneID Plate Position Well Length GeneSymbol GeneID Accession Description Vector 692672 HsxXG013989 2 B01 STK32A 202374 null serine/threonine kinase 32A pANT7_cGST 692675 HsxXG013989 3 C01 RPS10-NUDT3 100529239 null RPS10-NUDT3 readthrough pANT7_cGST 692678 HsxXG013989 4 D01 SPATA6L 55064 null spermatogenesis associated 6-like pANT7_cGST 692679 HsxXG013989 5 E01 ATP1A4 480 null ATPase, Na+/K+ transporting, alpha 4 polypeptide pANT7_cGST 692689 HsxXG013989 6 F01 ZNF816-ZNF321P 100529240 null ZNF816-ZNF321P readthrough pANT7_cGST 692691 HsxXG013989 7 G01 NKAIN1 79570 null Na+/K+ transporting ATPase interacting 1 pANT7_cGST 693155 HsxXG013989 8 H01 TNFSF12-TNFSF13 407977 NM_172089 TNFSF12-TNFSF13 readthrough pANT7_cGST 693161 HsxXG013989 9 A02 RAB12 201475 NM_001025300 RAB12, member RAS oncogene family pANT7_cGST 693169 HsxXG013989 10 B02 SYN1 6853 NM_133499 synapsin I pANT7_cGST 693176 HsxXG013989 11 C02 GJD3 125111 NM_152219 gap junction protein, delta 3, 31.9kDa pANT7_cGST 693181 HsxXG013989 12 D02 CHCHD10 400916 null coiled-coil-helix-coiled-coil-helix domain containing 10 pANT7_cGST 693184 HsxXG013989 13 E02 IDNK 414328 null idnK, gluconokinase homolog (E. coli) pANT7_cGST 693187 HsxXG013989 14 F02 LYPD6B 130576 null LY6/PLAUR domain containing 6B pANT7_cGST 693189 HsxXG013989 15 G02 C8orf86 389649 null chromosome 8 open reading frame 86 pANT7_cGST 693194 HsxXG013989 16 H02 CENPQ 55166 -

New and Future Drug Development for Gastroesophageal Reflux Disease
J Neurogastroenterol Motil, Vol. 20 No. 1 January, 2014 pISSN: 2093-0879 eISSN: 2093-0887 http://dx.doi.org/10.5056/jnm.2014.20.1.6 JNM Journal of Neurogastroenterology and Motility Review New and Future Drug Development for Gastroesophageal Reflux Disease Carla Maradey-Romero and Ronnie Fass* The Esophageal and Swallowing Center, Division of Gastroenterology and Hepatology, MetroHealth Medical Center, Case Western Reserve University, Cleveland, Ohio, USA Medical therapy remains the most popular treatment for gastroesophageal reflux disease (GERD). Whilst interest in drug devel- opment for GERD has declined over the last few years primarily due to the conversion of most proton pump inhibitor (PPI)’s to generic and over the counter compounds, there are still numerous areas of unmet needs in GERD. Drug development has been focused on potent histamine type 2 receptor antagonist’s, extended release PPI’s, PPI combination, potassium-competitive acid blockers, transient lower esophageal sphincter relaxation reducers, prokinetics, mucosal protectants and esophageal pain modulators. It is likely that the aforementioned compounds will be niched for specific areas of unmet need in GERD, rather than compete with the presently available anti-reflux therapies. (J Neurogastroenterol Motil 2014;20:6-16) Key Words Erosive esophagitis; Gastroesophageal reflux; Heartburn; Proton pump inhibitors Most patients with GERD fall into 1 of 3 categories: non- erosive reflux disease (NERD), erosive esophagitis (EE), and Introduction Barrett’s esophagus (BE). The 2 main phenotypes of GERD, Gastroesophageal reflux disease (GERD) is a common con- NERD and EE, appear to have different pathophysiological and dition that develops when reflux of stomach contents cause trou- clinical characteristics. -

Structural and Functional Dynamics of Serotonin Transporter Gene Variants
STRUCTURAL AND FUNCTIONAL DYNAMICS OF SEROTONIN TRANSPORTER GENE VARIANTS By Meagan Anne Quinlan Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University In partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY in Pharmacology May 10, 2019 Nashville, Tennessee Approved: Brian Wadzinski, PhD Heidi Hamm, PhD Kevin Schey, PhD Randy Blakely, PhD ACKNOWLEDGEMENTS The completion of this dissertation would not be possible without the unwavering support of several people that have helped me immensely along the way. I first must give my greatest acknowledgment to my Ph.D. mentor Dr. Randy Blakely, without whom this accomplishment would not be possible. Being able to research the serotonin transporter, the protein he cloned back in 1989, has been the highest honor. His depth of knowledge and his unwavering support was unmatched during all the experiments I thought failed. He did always find that silver lining in the blank blots. The enthusiasm he gleaned when I would have exciting new data and even the countless hours we discussed the nuances of serotonin transporter kinetics as we trialed our way through data that didn’t always make sense, will definitely not be forgotten. Needless to say, there was not a better mentor that could have taken me through this journey. I was also lucky to have a supportive thesis committee. To Dr. Brain Wadzinski, Dr. Heidi Hamm, Dr. Ana Carnerio, and Dr. Kevin Schey, all this work definitely would have not been possible without all your insightful questions and constructive critiques. Thank you all for your encouragement and help over the past years.