Atypical Nevi When to Re-Excise
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Features of Benign Tumors of the External Auditory Canal According to Pathology
Central Annals of Otolaryngology and Rhinology Research Article *Corresponding author Jae-Jun Song, Department of Otorhinolaryngology – Head and Neck Surgery, Korea University College of Clinical Features of Benign Medicine, 148 Gurodong-ro, Guro-gu, Seoul, 152-703, South Korea, Tel: 82-2-2626-3191; Fax: 82-2-868-0475; Tumors of the External Auditory Email: Submitted: 31 March 2017 Accepted: 20 April 2017 Canal According to Pathology Published: 21 April 2017 ISSN: 2379-948X Jeong-Rok Kim, HwibinIm, Sung Won Chae, and Jae-Jun Song* Copyright Department of Otorhinolaryngology-Head and Neck Surgery, Korea University College © 2017 Song et al. of Medicine, South Korea OPEN ACCESS Abstract Keywords Background and Objectives: Benign tumors of the external auditory canal (EAC) • External auditory canal are rare among head and neck tumors. The aim of this study was to analyze the clinical • Benign tumor features of patients who underwent surgery for an EAC mass confirmed as a benign • Surgical excision lesion. • Recurrence • Infection Methods: This retrospective study involved 53 patients with external auditory tumors who received surgical treatment at Korea University, Guro Hospital. Medical records and evaluations over a 10-year period were examined for clinical characteristics and pathologic diagnoses. Results: The most common pathologic diagnoses were nevus (40%), osteoma (13%), and cholesteatoma (13%). Among the five pathologic subgroups based on the origin organ of the tumor, the most prevalent pathologic subgroup was the skin lesion (47%), followed by the epithelial lesion (26%), and the bony lesion (13%). No significant differences were found in recurrence rate, recurrence duration, sex, or affected side between pathologic diagnoses. -

CASE REPORT Intradermal Nevus of the External Auditory Canal
Int. Adv. Otol. 2009; 5:(3) 401-403 CASE REPORT Intradermal Nevus of the External Auditory Canal: A Case Report Sedat Ozturkcan, Ali Ekber, Riza Dundar, Filiz Gulustan, Demet Etit, Huseyin Katilmis Department of Otorhinolaryngology and Head and Neck Surgery ‹zmir Atatürk Research and Training Hospital, Ministry of Health, ‹ZM‹R-TURKEY (SO, AE, FG, DE, HK) Department of Otorhinolaryngology and Head and Neck Surgery Etimesgut Military Hospital , ANKARA-TURKEY (RD) Intradermal nevus is the most common skin tumor in humans; however, its occurrence in the external auditory canal (EAC) is uncommon. The clinical manifestations of pigmented nevus of the EAC have been reported to include ear fullness, foreign body sensation, hearing impairment, and otalgia, but some cases were asymptomatic and were found incidentally. The treatment of choice for a symptomatic intradermal nevus in the EAC is complete excision. There has been no recurrence reported in the literature . A pedunculated, papillomatous hair-bearing lesion was detected in the external auditory canal of the patient who was on follow-up for pruritus. Clinical and pathologic features of an intradermal nevus of the external auditory canal are presented, and the literature reviewed. Submitted : 14 October 2008 Revised : 01 July 2009 Accepted : 09 July 2009 Intradermal nevus is the most common skin tumor in left external auditory canal. Otomicroscopic humans; however, its occurrence in the external examination revealed a pedunculated, papillomatous auditory canal (EAC) is uncommon [1-4]. Intradermal hair-bearing lesion in the postero-inferior cartilaginous nevus is considered to be a form of benign cutaneous portion of the external auditory canal (Figure 1). -

Acral Compound Nevus SJ Yun S Korea
University of Pennsylvania, Founded by Ben Franklin in 1740 Disclosures Consultant for Myriad Genetics and for SciBase (might try to sell you a book, as well) Multidimensional Pathway Classification of Melanocytic Tumors WHO 4th Edition, 2018 Epidemiologic, Clinical, Histologic and Genomic Aspects of Melanoma David E. Elder, MB ChB, FRCPA University of Pennsylvania, Philadelphia, PA, USA Napa, May, 2018 3rd Edition, 2006 Malignant Melanoma • A malignant tumor of melanocytes • Not all melanomas are the same – variation in: – Epidemiology – risk factors, populations – Cell/Site of origin – Precursors – Clinical morphology – Microscopic morphology – Simulants – Genomic abnormalities Incidence of Melanoma D.M. Parkin et al. CSD/Site-Related Classification • Bastian’s CSD/Site-Related Classification (Taxonomy) of Melanoma – “The guiding principles for distinguishing taxa are genetic alterations that arise early during progression; clinical or histologic features of the primary tumor; characteristics of the host, such as age of onset, ethnicity, and skin type; and the role of environmental factors such as UV radiation.” Bastian 2015 Epithelium associated Site High UV Low UV Glabrous Mucosa Benign Acquired Spitz nevus nevus Atypical Dysplastic Spitz Borderline nevus tumor High Desmopl. Low-CSD Spitzoid Acral Mucosal Malignant CSD melanoma melanoma melanoma melanoma melanoma 105 Point mutations 103 Structural Rearrangements 2018 WHO Classification of Melanoma • Integrates Epidemiologic, Genomic, Clinical and Histopathologic Features • Assists -
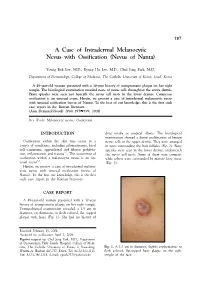
A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta)
197 A Case of Intradermal Melanocytic Nevus with Ossification (Nevus of Nanta) Young Bok Lee, M.D., Kyung Ho Lee, M.D., Chul Jong Park, M.D. Department of Dermatology, College of Medicine, The Catholic University of Korea, Seoul, Korea A 49-year-old woman presented with a 30-year history of asymptomatic plaque on her right temple. The histological examination revealed nests of nevus cells throughout the entire dermis. Bony spicules were seen just beneath the nevus cell nests in the lower dermis. Cutaneous ossification is an unusual event. Herein, we present a case of intradermal melanocytic nevus with unusual ossification (nevus of Nanta). To the best of our knowledge, this is the first such case report in the Korean literature. (Ann Dermatol (Seoul) 20(4) 197∼199, 2008) Key Words: Melanocytic nevus, Ossification INTRODUCTION drug intake or medical illness. The histological examination showed a dense proliferation of benign Ossification within the skin may occur in a nevus cells in the upper dermis. They were arranged variety of conditions, including pilomatricoma, basal in nests surrounding the hair follicles (Fig. 2). Bony cell carcinoma, appendageal and fibrous prolifera- spicules were seen in the lower dermis, underneath 1,2 tion, inflammation and trauma . The occurrence of the nevus cell nests. Some of them were compact ossification within a melanocytic nevus is an un- while others were surrounded by mature fatty tissue 3-5 usual event . (Fig. 3). Herein, we present a case of intradermal melano- cytic nevus with unusual ossification (nevus of Nanta). To the best our knowledge, this is the first such case report in the Korean literature. -

Acquired Bilateral Nevus of Ota–Like Macules (Hori's Nevus): a Case
Acquired Bilateral Nevus of Ota–like Macules (Hori’s Nevus): A Case Report and Treatment Update Jamie Hale, DO,* David Dorton, DO,** Kaisa van der Kooi, MD*** *Dermatology Resident, 2nd year, Largo Medical Center/NSUCOM, Largo, FL **Dermatologist, Teaching Faculty, Largo Medical Center/NSUCOM, Largo, FL ***Dermatopathologist, Teaching Faculty, Largo Medical Center/NSUCOM, Largo, FL Abstract This is a case of a 71-year-old African American female who presented with bilateral periorbital hyperpigmentation. After failing treatment with a topical retinoid and hydroquinone, a biopsy was performed and was consistent with acquired bilateral nevus of Ota-like macules, or Hori’s nevus. A review of histopathology, etiology, and treatment is discussed below. cream and tretinoin 0.05% gel. At this visit, a Introduction Figure 2 Acquired nevus of Ota-like macules (ABNOM), punch biopsy of her left zygoma was performed. or Hori’s nevus, clinically presents as bilateral, Histopathology reported sparse proliferation blue-gray to gray-brown macules of the zygomatic of irregularly shaped, haphazardly arranged melanocytes extending from the superficial area. It less often presents on the forehead, upper reticular dermis to mid-deep reticular dermis outer eyelids, and nose.1 It is most common in women of Asian descent and has been reported Figure 4 in ages 20 to 70. Classically, the eye and oral mucosa are uninvolved. This condition is commonly misdiagnosed as melasma.1 The etiology of this condition is not fully understood, and therefore no standardized treatment has been Figure 3 established. Case Report A 71-year-old African American female initially presented with a two week history of a pruritic, flaky rash with discoloration of her face. -

Short Course 11 Pigmented Lesions of the Skin
Rev Esp Patol 1999; Vol. 32, N~ 3: 447-453 © Prous Science, SA. © Sociedad Espafiola de Anatomfa Patol6gica Short Course 11 © Sociedad Espafiola de Citologia Pigmented lesions of the skin Chairperson F Contreras Spain Ca-chairpersons S McNutt USA and P McKee, USA. Problematic melanocytic nevi melanin pigment is often evident. Frequently, however, the lesion is solely intradermal when it may be confused with a fibrohistiocytic RH. McKee and F.R.C. Path tumor, particularly epithelloid cell fibrous histiocytoma (4). It is typi- cally composed of epitheliold nevus cells with abundant eosinophilic Brigham and Women’s Hospital, Harvard Medical School, Boston, cytoplasm and large, round, to oval vesicular nuclei containing pro- USA. minent eosinophilic nucleoli. Intranuclear cytoplasmic pseudoinclu- sions are common and mitotic figures are occasionally present. The nevus cells which are embedded in a dense, sclerotic connective tis- Whether the diagnosis of any particular nevus is problematic or not sue stroma, usually show maturation with depth. Less frequently the nevus is composed solely of spindle cells which may result in confu- depends upon a variety of factors, including the experience and enthusiasm of the pathologist, the nature of the specimen (shave vs. sion with atrophic fibrous histiocytoma. Desmoplastic nevus can be distinguished from epithelloid fibrous histiocytoma by its paucicellu- punch vs. excisional), the quality of the sections (and their staining), larity, absence of even a focal storiform growth pattern and SiQO pro- the hour of the day or day of the week in addition to the problems relating to the ever-increasing range of histological variants that we tein/HMB 45 expression. -

Optimal Management of Common Acquired Melanocytic Nevi (Moles): Current Perspectives
Clinical, Cosmetic and Investigational Dermatology Dovepress open access to scientific and medical research Open Access Full Text Article REVIEW Optimal management of common acquired melanocytic nevi (moles): current perspectives Kabir Sardana Abstract: Although common acquired melanocytic nevi are largely benign, they are probably Payal Chakravarty one of the most common indications for cosmetic surgery encountered by dermatologists. With Khushbu Goel recent advances, noninvasive tools can largely determine the potential for malignancy, although they cannot supplant histology. Although surgical shave excision with its myriad modifications Department of Dermatology and STD, Maulana Azad Medical College and has been in vogue for decades, the lack of an adequate histological sample, the largely blind Lok Nayak Hospital, New Delhi, Delhi, nature of the procedure, and the possibility of recurrence are persisting issues. Pigment-specific India lasers were initially used in the Q-switched mode, which was based on the thermal relaxation time of the melanocyte (size 7 µm; 1 µsec), which is not the primary target in melanocytic nevus. The cluster of nevus cells (100 µm) probably lends itself to treatment with a millisecond laser rather than a nanosecond laser. Thus, normal mode pigment-specific lasers and pulsed ablative lasers (CO2/erbium [Er]:yttrium aluminum garnet [YAG]) are more suited to treat acquired melanocytic nevi. The complexities of treating this disorder can be overcome by following a structured approach by using lasers that achieve the appropriate depth to treat the three subtypes of nevi: junctional, compound, and dermal. Thus, junctional nevi respond to Q-switched/normal mode pigment lasers, where for the compound and dermal nevi, pulsed ablative laser (CO2/ Er:YAG) may be needed. -

Nonpigmented Metastatic Melanoma in a Two-Year-Old Girl: a Serious Diagnostic Dilemma
Hindawi Publishing Corporation Case Reports in Oncological Medicine Volume 2015, Article ID 298273, 3 pages http://dx.doi.org/10.1155/2015/298273 Case Report Nonpigmented Metastatic Melanoma in a Two-Year-Old Girl: A Serious Diagnostic Dilemma Gulden Diniz,1 Hulya Tosun Yildirim,2 Selcen Yamaci,2 and Nur Olgun3 1 Izmir Tepecik Education and Research Hospital, Pathology Laboratory, Turkey 2Izmir Dr. Behcet Uz Children’s Hospital, Pathology Laboratory and Dermatology Clinics, Turkey 3Pediatric Oncology Clinics, Izmir Dokuz Eylul University, Turkey Correspondence should be addressed to Gulden Diniz; [email protected] Received 23 July 2014; Revised 20 January 2015; Accepted 21 January 2015 Academic Editor: Francesca Micci Copyright © 2015 Gulden Diniz et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Although rare, malignant melanoma may occur in children. Childhood melanomas account for only 0.3–3% of all melanomas. In particular the presence of congenital melanocytic nevi is associated with an increased risk of development of melanoma. We herein report a case of malignant melanoma that developed on a giant congenital melanocytic nevus and made a metastasis to the subcutaneous tissue of neck in a two-year-old girl. The patient was hospitalized for differential diagnosis and treatment of cervical mass with a suspicion of hematological malignancy, because the malignant transformation of congenital nevus was not noticed before. In this case, we found out a nonpigmented malignant tumor of pleomorphic cells after the microscopic examination of subcutaneous lesion. -

Dermatopathology
Dermatopathology Clay Cockerell • Martin C. Mihm Jr. • Brian J. Hall Cary Chisholm • Chad Jessup • Margaret Merola With contributions from: Jerad M. Gardner • Talley Whang Dermatopathology Clinicopathological Correlations Clay Cockerell Cary Chisholm Department of Dermatology Department of Pathology and Dermatopathology University of Texas Southwestern Medical Center Central Texas Pathology Laboratory Dallas , TX Waco , TX USA USA Martin C. Mihm Jr. Chad Jessup Department of Dermatology Department of Dermatology Brigham and Women’s Hospital Tufts Medical Center Boston , MA Boston , MA USA USA Brian J. Hall Margaret Merola Department of Dermatology Department of Pathology University of Texas Southwestern Medical Center Brigham and Women’s Hospital Dallas , TX Boston , MA USA USA With contributions from: Jerad M. Gardner Talley Whang Department of Pathology and Dermatology Harvard Vanguard Medical Associates University of Arkansas for Medical Sciences Boston, MA Little Rock, AR USA USA ISBN 978-1-4471-5447-1 ISBN 978-1-4471-5448-8 (eBook) DOI 10.1007/978-1-4471-5448-8 Springer London Heidelberg New York Dordrecht Library of Congress Control Number: 2013956345 © Springer-Verlag London 2014 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifi cally the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfi lms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. Exempted from this legal reservation are brief excerpts in connection with reviews or scholarly analysis or material supplied specifi cally for the purpose of being entered and executed on a computer system, for exclusive use by the purchaser of the work. -

Melanomas Are Comprised of Multiple Biologically Distinct Categories
Melanomas are comprised of multiple biologically distinct categories, which differ in cell of origin, age of onset, clinical and histologic presentation, pattern of metastasis, ethnic distribution, causative role of UV radiation, predisposing germ line alterations, mutational processes, and patterns of somatic mutations. Neoplasms are initiated by gain of function mutations in one of several primary oncogenes, typically leading to benign melanocytic nevi with characteristic histologic features. The progression of nevi is restrained by multiple tumor suppressive mechanisms. Secondary genetic alterations override these barriers and promote intermediate or overtly malignant tumors along distinct progression trajectories. The current knowledge about pathogenesis, clinical, histological and genetic features of primary melanocytic neoplasms is reviewed and integrated into a taxonomic framework. THE MOLECULAR PATHOLOGY OF MELANOMA: AN INTEGRATED TAXONOMY OF MELANOCYTIC NEOPLASIA Boris C. Bastian Corresponding Author: Boris C. Bastian, M.D. Ph.D. Gerson & Barbara Bass Bakar Distinguished Professor of Cancer Biology Departments of Dermatology and Pathology University of California, San Francisco UCSF Cardiovascular Research Institute 555 Mission Bay Blvd South Box 3118, Room 252K San Francisco, CA 94158-9001 [email protected] Key words: Genetics Pathogenesis Classification Mutation Nevi Table of Contents Molecular pathogenesis of melanocytic neoplasia .................................................... 1 Classification of melanocytic neoplasms -

A Nevus of OTA with Intraoral Involvement: a Rare Case Report
Shivare P. et al.: Nevus of OTA- a rare entity CASE REPORT A Nevus of OTA with Intraoral Involvement: A Rare Case Report Peeyush Shivhare1, Lata S.2, Monu Yadav3, Naqoosh Haidry4, Shruthi T. Patil5 1,5- Senior lecturer department of oral medicine and radiology, Narsinhbhai patel dental college and hospital, Visnagar, Gujarat. 2- Professor and head of the department, Rungta Correspondence to: College Of Dental Sciences And Research, Bhilai, Chhattisgarh. 3- PG student. Dr. Peeyush Shivhare Senior lecturer department of Department Of Oral Medicine And Radiology, Carrier Dental College And Hospital. oral medicine and radiology, Narsinhbhai patel dental Lucknow. 4- Senior lecturer department of maxillofacial surgery, Narsinhbhai patel college and hospital, Visnagar, Gujarat. dental college and hospital, Visnagar, Gujarat. Contact Us: www.ijohmr.com ABSTRACT Nevus of Ota, which originally was described by Ota and Tanino in 1939. It is characterized as congenital or acquired hamartoma of dermal melanocytes, presents clinically as a blue or gray patch on the face within the distribution of the ophthalmic and maxillary branches of the fifth cranial (trigeminal) nerve. Involvement of the palatal mucosa occurs rarely in nevus of Ota, when it occurs, it usually blends with the oral mucosa and is typically irregular, ill defined and often present as a mottled patch. Nevus of Ota is rare in the Indian subcontinent. So far very less cases of nevus of ota with intraoral involvement have been documented in the English literature. We report a rare case of intraoral nevus of Ota in a 20 year-old female patient. KEYWORDS: Nevus of Ota, Melanoma, Hamartoma, Glaucoma AA aaaasasasss INTRODUCTION The nevus of Ota (nevus fuscoceruleus ophthal- momaxillaris” or oculodermal melanocytosis) is a macular discoloration of the face, found most commonly in the Japanese people.1 Nevus of ota develops when the melanocyte get entrapped in the upper third of the dermis. -

Superficial Melanocytic Pathology Melanocytic Superficial Atypical Melanocytic Proliferations Pathology Superficial Atypical David E
Consultant Consultant Pathology Series Editor ■ David E. Elder, MB, ChB Series Editor ■ David E. Elder, MB, ChB ■ Pathology7 Consultant Pathology 7 MelanocyticSuperficial Pathology 7 Superficial Superficial Melanocytic Pathology Melanocytic Superficial Atypical Melanocytic Proliferations Pathology Superficial Atypical David E. Elder, MB, ChB • Sook Jung Yun, MD, PhD Melanocytic Proliferations Add Expert Analysis of Difficult Cases to Your Practice With Consultant Pathology Superficial Melanocytic Pathology provides expert guidance for resolving the real world problems pathologists face when diagnosing melanomas and other atypical pigmented lesions. It reviews each major category of atypical melanocytic lesions, including the pathology of the superficial categories of melanoma followed by a discussion of the major simulants David E. Elder of melanoma. The book provides an overview of the morphologic description and diagnostic issues for each lesion, followed by 60 detailed, Sook Jung Yun abundantly illustrated case presentations, offering an expert approach to diagnosis for a range of challenging cases. Five hundred high-quality color images support the case presentations. Of special interest is a chapter on “ambiguous” lesions of uncertain significance. The book’s thorough analysis of challenging lesions will aid pathologists in differentiating between benign superficial proliferations and malignant melanocytic tumors. All Consultant Pathology Titles Provide: Elder • Yun ■ ACTUAL consultation cases and expert analysis ■ EXPERT analysis